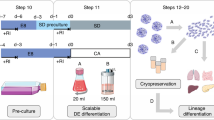
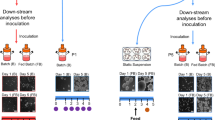
Abstract
A promising cell-therapy approach for heart failure aims at differentiating human pluripotent stem cells (hPSCs) into functional cardiomyocytes (CMs) in vitro to replace the disease-induced loss of patients’ heart muscle cells in vivo. But many challenges remain for the routine clinical application of hPSC-derived CMs (hPSC-CMs), including good manufacturing practice (GMP)-compliant production strategies. This protocol describes the efficient generation of hPSC-CM aggregates in suspension culture, emphasizing process simplicity, robustness and GMP compliance. The strategy promotes clinical translation and other applications that require large numbers of CMs. Using a simple spinner-flask platform, this protocol is applicable to a broad range of users with general experience in handling hPSCs without extensive know-how in biotechnology. hPSCs are expanded in monolayer to generate the required cell numbers for process inoculation in suspension culture, followed by stirring-controlled formation of cell-only aggregates at a 300-ml scale. After 48 h at checkpoint (CP) 0, chemically defined cardiac differentiation is induced by WNT-pathway modulation through use of the glycogen-synthase kinase-3 inhibitor CHIR99021 (WNT agonist), which is replaced 24 h later by the chemical WNT-pathway inhibitor IWP-2. The exact application of the described process parameters is important to ensure process efficiency and robustness. After 10 d of differentiation (CP I), the production of ≥100 × 106 CMs is expected. Moreover, to ‘uncouple’ cell production from downstream applications, continuous maintenance of CM aggregates for up to 35 d in culture (CP II) is demonstrated without a reduction in CM content, supporting downstream logistics while potentially overcoming the requirement for cryopreservation.
Key points
-
We present a protocol for the efficient generation of hPSC-CM aggregates in suspension culture, emphasizing process simplicity, robustness and GMP compliance. The strategy promotes clinical translation and other applications that require large numbers of CMs.
-
This protocol uses a simple spinner-flask platform, making it accessible to users experienced in the handling of hPSCs but without extensive experience in biotechnology. This enables straightforward adaptation by many laboratories without bioprocessing experience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The flow cytometry data are available in the FlowRepository under accession code FR-FCM-Z6K3. All remaining data generated or analyzed during this study are included in this published article and its supplementary files.
References
Laflamme, M. A. & Murry, C. E. Heart regeneration. Nature 473, 326–335 (2011).
Kobold, S. et al. A manually curated database on clinical studies involving cell products derived from human pluripotent stem cells. Stem Cell Rep. 15, 546–555 (2020).
Ilic, D. & Ogilvie, C. Pluripotent stem cells in clinical setting—new developments and overview of current status. Stem Cells 40, 791–801 (2022).
Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557, 619–620 (2018).
Mallapaty, S. Revealed: two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 581, 249–250 (2020).
Silver, S. E., Barrs, R. W. & Mei, Y. Transplantation of human pluripotent stem cell-derived cardiomyocytes for cardiac regenerative therapy. Front. Cardiovasc. Med. 8, 707890 (2021).
Liu, Y.-W. et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
van den Akker, F. et al. Intramyocardial stem cell injection: go(ne) with the flow. Eur. Heart J. 38, 184–186 (2016).
Hogrebe, N. J., Maxwell, K. G., Augsornworawat, P. & Millman, J. R. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat. Protoc. 16, 4109–4143 (2021).
Preininger, M. K., Singh, M. & Xu, C. Cryopreservation of human pluripotent stem cell-derived cardiomyocytes: strategies, challenges, and future directions. Adv. Exp. Med. Biol. 951, 123–135 (2016).
Halloin, C. et al. Continuous WNT control enables advanced hPSC cardiac processing and prognostic surface marker identification in chemically defined suspension culture. Stem Cell Rep. 13, 366–379 (2019).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Fonoudi, H. et al. Large-scale production of cardiomyocytes from human pluripotent stem cells using a highly reproducible small molecule-based differentiation protocol. J. Vis. Exp. 2016, 54276 (2016).
Kahn-Krell, A. et al. Bioreactor suspension culture: differentiation and production of cardiomyocyte spheroids from human induced pluripotent stem cells. Front. Bioeng. Biotechnol. 9, 674260 (2021).
Kempf, H., Kropp, C., Olmer, R., Martin, U. & Zweigerdt, R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 10, 1345–1361 (2015).
Chen, A., Ting, S., Seow, J., Reuveny, S. & Oh, S. Considerations in designing systems for large scale production of human cardiomyocytes from pluripotent stem cells. Stem Cell Res. Ther. 5, 12 (2014).
Kempf, H. et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 3, 1132–1146 (2014).
Manstein, F. et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med 10, 1063–1080 (2021).
Langenberg, K. et al. Controlled stirred tank bioreactors for large-scale manufacture of human iPSC models for cell therapy. Cytotherapy 22, S43 (2020).
Fischer, B. et al. A complete workflow for the differentiation and the dissociation of hiPSC-derived cardiospheres. Stem Cell Res 32, 65–72 (2018).
Correia, C. et al. Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev. Rep. 10, 786–801 (2014).
Hamad, S. et al. Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations. Theranostics 9, 7222–7238 (2019).
Sahabian, A. et al. Chemically-defined, xeno-free, scalable production of hPSC-derived definitive endoderm aggregates with multi-lineage differentiation potential. Cells 8, 1571 (2019).
Ackermann, M. et al. Continuous human iPSC-macrophage mass production by suspension culture in stirred tank bioreactors. Nat. Protoc. 17, 513–539 (2022).
Chen, V. C. et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 15, 365–375 (2015).
Shafa, M., Panchalingam, K. M., Walsh, T., Richardson, T. & Baghbaderani, B. A. Computational fluid dynamics modeling, a novel, and effective approach for developing scalable cell therapy manufacturing processes. Biotechnol. Bioeng. 116, 3228–3241 (2019).
Kropp, C. et al. Impact of feeding strategies on the scalable expansion of human pluripotent stem cells in single-use stirred tank bioreactors. Stem Cells Transl. Med 5, 1289–1301 (2016).
Kempf, H. et al. Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells. Nat. Commun. 7, 13602 (2016).
Zweigerdt, R., Olmer, R., Singh, H., Haverich, A. & Martin, U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat. Protoc. 6, 689–700 (2011).
Olmer, R. et al. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng. Part C. Methods 18, 772–784 (2012).
Kiesslich, S. & Kamen, A. A. Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnol. Adv. 44, 107608 (2020).
Ashok, P., Parikh, A., Du, C. & Tzanakakis, E. S. Xenogeneic-free system for biomanufacturing of cardiomyocyte progeny from human pluripotent stem cells. Front. Bioeng. Biotechnol. 8, 571425 (2020).
Manstein, F. et al. Protocol process control and in silico modeling strategies for enabling high density culture of human pluripotent stem cells in stirred tank bioreactors. STAR Protoc. 2, 100988 (2021).
Gaspari, E. et al. Paracrine mechanisms in early differentiation of human pluripotent stem cells: insights from a mathematical model. Stem Cell Res. 32, 1–7 (2018).
Williams, B. et al. Prediction of human induced pluripotent stem cell cardiac differentiation outcome by multifactorial process modeling. Front. Bioeng. Biotechnol. 8, 851 (2020).
Floy, M. E. et al. Advances in manufacturing cardiomyocytes from human pluripotent stem cells. Annu. Rev. Chem. Biomol. Eng. 13, 255–278 (2022).
Kattman, S. J. et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 (2011).
Lundy, S. D., Zhu, W. Z., Regnier, M. & Laflamme, M. A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 22, 1991–2002 (2013).
Sartiani, L. et al. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells 25, 1136–1144 (2007).
Wickramasinghe, N. M. et al. PPARdelta activation induces metabolic and contractile maturation of human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 29, 559–576.e7 (2022).
Krüger, M. et al. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/ AKT pathway. Circ. Res. 102, 439–447 (2008).
Yang, X. et al. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 72, 296–304 (2014).
Rog-Zielinska, E. A. et al. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1α. Cell Death Differ. 22, 1106–1116 (2015).
Parikh, S. S. et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell–derived cardiomyocytes. Circ. Res. 121, 1323–1330 (2017).
Correia, C. et al. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 7, 8590 (2017).
Yang, X. et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 13, 657–668 (2019).
Paredes, A. et al. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature 618, 365–373 (2023).
Peters, M. C. et al. Metabolic maturation increases susceptibility to hypoxia-induced damage in human iPSC-derived cardiomyocytes. Stem Cells Transl. Med. 11, 1040–1051 (2022).
Haase, A. et al. GMP-compatible manufacturing of three iPS cell lines from human peripheral blood. Stem Cell Res. 35, 101394 (2019).
Papoutsakis, E. T. Media additives for protecting freely suspended animal cells against agitation and aeration damage. Trends Biotechnol. 9, 316–324 (1991).
Zhang, Z., Al-Rubeai, M. & Thomas, C. R. Effect of Pluronic F-68 on the mechanical properties of mammalian cells. Enzym. Microb. Technol. 14, 980–983 (1992).
Haase, A., Göhring, G. & Martin, U. Generation of non-transgenic iPS cells from human cord blood CD34+ cells under animal component-free conditions. Stem Cell Res. 21, 71–73 (2017).
Haase, A. et al. Establishment of MHHi001-A-5, a GCaMP6f and RedStar dual reporter human iPSC line for in vitro and in vivo characterization and in situ tracing of iPSC derivatives. Stem Cell Res. 52, 102206 (2021).
Drakhlis, L., Devadas, S. B. & Zweigerdt, R. Generation of heart-forming organoids from human pluripotent stem cells. Nat. Protoc. 16, 5652–5672 (2021).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Zhu, W.-Z., Van Biber, B. & Laflamme, M. A. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol. Biol. 767, 419–431 (2011).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Cambier, L., Plate, M., Sucov, H. M. & Pashmforoush, M. Nkx2-5 regulates cardiac growth through modulation of Wnt signaling by R-spondin3. Development 141, 2959–2971 (2014).
Tarbit, E., Singh, I., Peart, J. N. & Rose’Meyer, R. B. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail. Rev. 24, 1–15 (2019).
Novak, D. et al. SOX2 in development and cancer biology. Semin. Cancer Biol. 67, 74–82 (2020).
Lu, H., Ma, J., Yang, Y., Shi, W. & Luo, L. EpCAM is an endoderm-specific Wnt derepressor that licenses hepatic development. Dev. Cell 24, 543–553 (2013).
Lertkiatmongkol, P., Liao, D., Mei, H., Hu, Y. & Newman, P. J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 23, 253–259 (2016).
Hamad, S. et al. High-efficient serum-free differentiation of endothelial cells from human iPS cells. Stem Cell Res. Ther. 13, 251 (2022).
Acknowledgements
This work was supported by the German Research Foundation (DFG; grants Cluster of Excellence REBIRTH EXC 62/2 and ZW64/4-2), the Federal Ministry of Education and Research (BMBF; grants 01EK1601A, 13XP5092B, 031L0249 and 01EK2108A), Lower Saxony ‘Förderung aus Mitteln des Niedersächsischen Vorab’ (grant ZN3340) and ‘Niedersächsische Ministerium für Wissenschaft und Kultur’ (MWK; grant ZN4092) and the European Union (Horizon Europe project HEAL grant 101056712). The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them. We thank R. Bauerfeind and O. Terwolbeck from the MHH Core Unit for laser microscopy and for help with confocal microscopy.
Author information
Authors and Affiliations
Contributions
N.K., W.T., C.H., U.M. and R.Z. designed the experiments. N.K., W.T., C.H., M.M., A.F., L.D. and J.T. contributed to the experimental design, performed the experiments and analyzed the data. A.H. generated hiPSC lines. F.M., K.U. and C.H. developed scripts for automatized analysis of the experiments. N.K., W.T., U.M. and R.Z. wrote and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.H. is an employee of Novo Nordisk. F.M. and W.T. are employees of Evotec. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Kurt Pfannkuche and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Halloin, C. et al. Stem Cell Rep. 13, 366–379 (2019): https://doi.org/10.1016/j.stemcr.2019.09.001
Extended data
Extended Data Fig. 1 Exemplary production of hiPSC-derived cardiomyocytes from vitronectin and (GMP-conforming) CTS vitronectin pre-culture.
a and b, Appearance of cells on CTS vitronectin (a) and vitronectin (b) on day −2 of the production protocol in monolayer (scale bar, 500 µm). c, Expression of markers of an undifferentiated state at CP 0 before induction of differentiation in samples from spinner flasks pre-cultured on vitronectin or CTS vitronectin. d, Cell yield at CP I of respective spinner-flask runs. e, Aggregate diameter of representative samples taken at CP I from respective spinners. Shown are individual values and mean ± s.d. in red. f, Percentage of cells positive for cardiac markers in representative samples from the respective spinner flasks at CP I.
Extended Data Fig. 2 Setup of the spinner flask.
In a sterile environment, unpack from plastic and install the lid with the stirrer. Remove one of the lids for the side ports and install a sampling device (optional).
Extended Data Fig. 3 Aggregate diameter size distribution for one differentiation run at CP 0, CP I and CP II.
Shown are individual aggregate diameters (gray) and mean values ± s.d. (red). The hiPSC line used is Amber.
Extended Data Fig. 4 Total aggregate count per milliliter of spinner flask experiments at CP 0 (n = 3) and CP I (n = 2).
Aggregate numbers were determined through dilution of a culture sample and manual counting through microscopy.
Extended Data Fig. 5 Confocal microscopy of aggregates at CP 0 and CP I to analyze cell number per aggregate.
a–c, Light microscope pictures of a representative cell sample at CP 0 (scale bar, 500 µm) (a) and confocal images of the central Z-stack of aggregates of the same batch (scale bar, 50 µm) (b and c). d–f, Light microscope pictures of a representative cell sample at CP I (scale bar, 500 µm) (d) and confocal images in a differentiated state as CM aggregates at CP I (scale bar, 50 µm) (e and f). Nuclei were stained with Sytox red after fixation; afterwards, aggregates were dehydrated with ethanol and cleared with methyl salicylate/benzyl benzoate (MSBB). Notable are pronounced cavities at CP 0 as well as at CP I. g, Aggregates treated for confocal imaging do not differ in diameter compared to untreated, equivalent samples measured according to QC3 at CP 0 or CP I. h, Nuclei identified at CP 0 and CP I through automatic counting of confocal z-stacks imaging whole aggregates. This nuclei count should closely resemble the total cell count per aggregate. i and j, Identified nuclei per aggregate in comparison to the individual aggregate diameter for aggregates at CP 0 (i) and CP I (j). Linear regression was added to depict the goodness of fit (CP 0 R2= 0.73; CP I R2= 0.55). For examples, see supplementary file z-stacks for CP 0 and CP I (open with the hyperstack function of FIJI/ImageJ). PFA, paraformaldehyde. The hiPSC line Phoenix was used in all experiments.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2
Supplementary Video 1
CM harvest for downstream application
Supplementary Video 2
hPSC splitting
Supplementary Video 3
Spinner medium exchange
Supplementary Data 1
Confocal z-stack pluripotent aggregates CP 0
Supplementary Data 2
Confocal z-stack CM aggregates CP I
Supplementary Code 1
ImageJ macro for aggregate size determination
Supplementary Code 2
ImageJ macro for viability score determination
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kriedemann, N., Triebert, W., Teske, J. et al. Standardized production of hPSC-derived cardiomyocyte aggregates in stirred spinner flasks. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00976-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00976-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.