Abstract
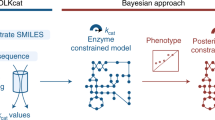
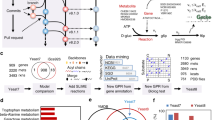
Genome-scale metabolic models (GEMs) are computational representations that enable mathematical exploration of metabolic behaviors within cellular and environmental constraints. Despite their wide usage in biotechnology, biomedicine and fundamental studies, there are many phenotypes that GEMs are unable to correctly predict. GECKO is a method to improve the predictive power of a GEM by incorporating enzymatic constraints using kinetic and omics data. GECKO has enabled reconstruction of enzyme-constrained metabolic models (ecModels) for diverse organisms, which show better predictive performance than conventional GEMs. In this protocol, we describe how to use the latest version GECKO 3.0; the procedure has five stages: (1) expansion from a starting metabolic model to an ecModel structure, (2) integration of enzyme turnover numbers into the ecModel structure, (3) model tuning, (4) integration of proteomics data into the ecModel and (5) simulation and analysis of ecModels. GECKO 3.0 incorporates deep learning-predicted enzyme kinetics, paving the way for improved metabolic models for virtually any organism and cell line in the absence of experimental data. The time of running the whole protocol is organism dependent, e.g., ~5 h for yeast.
Key points
-
Genome-scale metabolic models have the potential to predict changes in phenotype resulting from different environmental conditions. Their predictive power can be improved by including more information about the enzyme kinetics.
-
GECKO is a program that gives users an automated and manual mechanism to incorporate relevant data. The protocol also describes how to tune the program to compensate for incorrect or missing values.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All (limited) data used in the tutorials are distributed as part of the code (see below).
Code availability
The source code of the GECKO toolbox is publicly available under the MIT license at: https://github.com/SysBioChalmers/GECKO/, also available at https://doi.org/10.5281/zenodo.7699818.
References
Kim, W. J., Kim, H. U. & Lee, S. Y. Current state and applications of microbial genome-scale metabolic models. Curr. Opin. Syst. Biol. 2, 10–18 (2017).
Chen, Y., Li, G. & Nielsen, J. Genome-scale metabolic modeling from yeast to human cell models of complex diseases: latest advances and challenges. Methods Mol. Biol. 2049, 329–345 (2019).
Clark, T. J., Guo, L., Morgan, J. & Schwender, J. Modeling plant metabolism: from network reconstruction to mechanistic models. Annu. Rev. Plant Biol. 71, 303–326 (2020).
Fang, X., Lloyd, C. J. & Palsson, B. O. Reconstructing organisms in silico: genome-scale models and their emerging applications. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-020-00440-4 (2020).
Heinken, A., Basile, A., Hertel, J., Thinnes, C. & Thiele, I. Genome-scale metabolic modeling of the human microbiome in the era of personalized medicine. Annu Rev. Microbiol 75, 199–222 (2021).
Lewis, N. E., Nagarajan, H. & Palsson, B. O. Constraining the metabolic genotype–phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 10, 291–305 (2012).
Lloyd, C. J. et al. COBRAme: a computational framework for genome-scale models of metabolism and gene expression. PLoS Comput. Biol. 14, e1006302 (2018).
Goelzer, A. et al. Quantitative prediction of genome-wide resource allocation in bacteria. Metab. Eng. 32, 232–243 (2015).
Salvy, P. & Hatzimanikatis, V. The ETFL formulation allows multi-omics integration in thermodynamics-compliant metabolism and expression models. Nat. Commun. 11, 30 (2020).
Chen, Y. et al. Proteome constraints reveal targets for improving microbial fitness in nutrient‐rich environments. Mol. Syst. Biol. 17, e10093 (2021).
Beg, Q. K. et al. Intracellular crowding defines the mode and sequence of substrate uptake by Escherichia coli and constrains its metabolic activity. Proc. Natl Acad. Sci. USA 104, 12663–12668 (2007).
Adadi, R., Volkmer, B., Milo, R., Heinemann, M. & Shlomi, T. Prediction of microbial growth rate versus biomass yield by a metabolic network with kinetic parameters. PLoS Comput. Biol. 8, e1002575 (2012).
Sánchez, B. J. et al. Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol. Syst. Biol. 13, 935 (2017).
Bekiaris, P. S. & Klamt, S. Automatic construction of metabolic models with enzyme constraints. BMC Bioinforma. 21, 19 (2020).
Mao, Z. et al. ECMpy, a simplified workflow for constructing enzymatic constrained metabolic network model. Biomolecules 12, 65 (2022).
Domenzain, I. et al. Reconstruction of a catalogue of genome-scale metabolic models with enzymatic constraints using GECKO 2.0. Nat. Commun. 13, 1–13 (2022).
Chen, Y. & Nielsen, J. Mathematical modelling of proteome constraints within metabolism. Curr. Opin. Syst. Biol. 25, 50–56 (2021).
Kerkhoven, E. J. Advances in constraint-based models: methods for improved predictive power based on resource allocation constraints. Curr. Opin. Microbiol. 68, 102168 (2022).
Chen, Y. & Nielsen, J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl Acad. Sci. USA 116, 17592–17597 (2019).
Gustafsson, J. et al. Metabolic collaboration between cells in the tumor microenvironment has a negligible effect on tumor growth. Preprint at bioRxiv https://doi.org/10.1101/2022.02.08.479584 (2022).
Chen, Y. & Nielsen, J. Yeast has evolved to minimize protein resource cost for synthesizing amino acids. Proc. Natl Acad. Sci. USA 119, e2114622119 (2022).
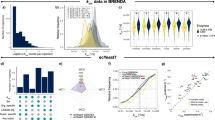
Chang, A. et al. BRENDA, the ELIXIR core data resource in 2021: new developments and updates. Nucleic Acids Res. 49, D498–D508 (2021).
Davidi, D. & Milo, R. Lessons on enzyme kinetics from quantitative proteomics. Curr. Opin. Biotechnol. 46, 81–89 (2017).
Li, F. et al. Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction. Nat. Catal. https://doi.org/10.1038/s41929-022-00798-z (2022).
Sulheim, S. et al. Enzyme-constrained models and omics analysis of Streptomyces coelicolor reveal metabolic changes that enhance heterologous production. iScience 23, 101525 (2020).
Zhou, J., Zhuang, Y. & Xia, J. Integration of enzyme constraints in a genome-scale metabolic model of Aspergillus niger improves phenotype predictions. Microb. Cell Fact. 20, 125 (2021).
Ye, C. et al. Improving lysine production through construction of an Escherichia coli enzyme‐constrained model. Biotechnol. Bioeng. 117, 3533–3544 (2020).
Chen, Y. et al. Genome‐scale modeling for Bacillus coagulans to understand the metabolic characteristics. Biotechnol. Bioeng. 117, 3545–3558 (2020).
Lu, H. et al. Yeast metabolic innovations emerged via expanded metabolic network and gene positive selection. Mol. Syst. Biol. 17, e10427 (2021).
Ishchuk, O. P. et al. Genome-scale modeling drives 70-fold improvement of intracellular heme production in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 119, e2108245119 (2022).
Li, Z. et al. Systems engineering of Escherichia coli for high-level shikimate production. Metab. Eng. 75, 1–11 (2023).
Bujdoš, D. et al. Engineering of Pseudomonas putida for accelerated co-utilization of glucose and cellobiose yields aerobic overproduction of pyruvate explained by an upgraded metabolic model. Metab. Eng. 75, 29–46 (2023).
Domenzain, I., Lu, Y., Shi, J., Lu, H. & Nielsen, J. Computational biology predicts metabolic engineering targets for increased production of 102 valuable chemicals in yeast. Preprint at bioRxiv https://doi.org/10.1101/2023.01.31.526512 (2023).
Heirendt, L. et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. https://doi.org/10.1038/s41596-018-0098-2 (2019).
Orth, J. D., Thiele, I. & Palsson, B. O. What is flux balance analysis? Nat. Biotechnol. 28, 245 (2010).
Gudmundsson, S. & Thiele, I. Computationally efficient flux variability analysis. BMC Bioinforma. 11, 489 (2010).
Choi, H. S., Lee, S. Y., Kim, T. Y. & Woo, H. M. In silico identification of gene amplification targets for improvement of lycopene production. Appl. Environ. Microbiol. 76, 3097–3105 (2010).
Agren, R. et al. Identification of anticancer drugs for hepatocellular carcinoma through personalized genome-scale metabolic modeling. Mol. Syst. Biol. 10, 721 (2014).
Cho, J. S., Gu, C., Han, T. H., Ryu, J. Y. & Lee, S. Y. Reconstruction of context-specific genome-scale metabolic models using multiomics data to study metabolic rewiring. Curr. Opin. Syst. Biol. 15, 1–11 (2019).
Robinson, J. L. et al. An atlas of human metabolism. Sci. Signal. 13, 1482 (2020).
Yeo, H. C., Hong, J., Lakshmanan, M. & Lee, D. Y. Enzyme capacity-based genome scale modelling of CHO cells. Metab. Eng. 60, 138–147 (2020).
Wendering, P. & Nikoloski, Z. Genome-scale modeling specifies the metabolic capabilities of Rhizophagus irregularis. mSystems 7, e0121621 (2022).
Wu, K. et al. ecBSU1: A genome-scale enzyme-constrained model of Bacillus subtilis based on the ECMpy workflow. Microorganisms 11, 178 (2023).
Österberg, L. et al. A novel yeast hybrid modeling framework integrating Boolean and enzyme-constrained networks enables exploration of the interplay between signaling and metabolism. PLoS Comput. Biol. 17, e1008891 (2021).
Chen, Y., Li, F. & Nielsen, J. Genome-scale modeling of yeast metabolism: retrospectives and perspectives. FEMS Yeast Res 22, 1–9 (2022).
The UniProt Consortium. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531 (2023).
Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M. & Tanabe, M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res 49, D545–D551 (2021).
Thiele, I. & Palsson, B. Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 5, 93–121 (2010).
Norsigian, C. J., Fang, X., Seif, Y., Monk, J. M. & Palsson, B. O. A workflow for generating multi-strain genome-scale metabolic models of prokaryotes. Nat. Protoc. https://doi.org/10.1038/s41596-019-0254-3 (2019).
Machado, D., Andrejev, S., Tramontano, M. & Patil, K. R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res 46, 7542–7553 (2018).
Wang, H. et al. RAVEN 2.0: a versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 14, e1006541 (2018).
Davidi, D. et al. Global characterization of in vivo enzyme catalytic rates and their correspondence to in vitro kcat measurements. Proc. Natl Acad. Sci. USA 113, 3401–3406 (2016).
Küken, A., Gennermann, K. & Nikoloski, Z. Characterization of maximal enzyme catalytic rates in central metabolism of Arabidopsis thaliana. Plant J. 103, 2168–2177 (2020).
Heckmann, D. et al. Kinetic profiling of metabolic specialists demonstrates stability and consistency of in vivo enzyme turnover numbers. Proc. Natl Acad. Sci. USA 117, 23182–23190 (2020).
Chen, Y. & Nielsen, J. In vitro turnover numbers do not reflect in vivo activities of yeast enzymes. Proc. Natl Acad. Sci. USA 118, 2021 (2021).
Xu, R., Razaghi-Moghadam, Z. & Nikoloski, Z. Maximization of non-idle enzymes improves the coverage of the estimated maximal in vivo enzyme catalytic rates in Escherichia coli. Bioinformatics https://doi.org/10.1093/BIOINFORMATICS/BTAB575 (2021).
Heckmann, D. et al. Machine learning applied to enzyme turnover numbers reveals protein structural correlates and improves metabolic models. Nat. Commun. 9, 5252 (2018).
Schendel, F., Mueller, E., Stubbe, J., Shiau, A. & Smith, J. Formylglycinamide ribonucleotide synthetase from Escherichia coli: cloning, sequencing, overproduction, isolation, and characterization. Biochemistry 28, 2459–2471 (1989).
Sánchez, B. J. et al. Benchmarking accuracy and precision of intensity-based absolute quantification of protein abundances in Saccharomyces cerevisiae. Proteomics 21, e2000093 (2021).
Van Hoek, P., Van Dijken, J. P. & Pronk, J. T. Effect of specific growth rate on fermentative capacity of baker’s yeast. Appl. Environ. Microbiol. 64, 4226–4233 (1998).
Heublein, M. et al. The novel component Kgd4 recruits the E3 subunit to the mitochondrial α-ketoglutarate dehydrogenase. Mol. Biol. Cell 25, 3342–3349 (2014).
Sánchez, B. J., Li, F., Kerkhoven, E. J. & Nielsen, J. SLIMEr: probing flexibility of lipid metabolism in yeast with an improved constraint-based modeling framework. BMC Syst. Biol. 13, 4 (2019).
Lewis, N. E. et al. Omic data from evolved E. coli are consistent with computed optimal growth from genome-scale models. Mol. Syst. Biol. 6, 390 (2010).
Acknowledgements
The Novo Nordisk Foundation (grant no. NNF20CC0035580); the Knut and Alice Wallenberg Foundation; the European Union’s Horizon 2020 research and innovation program (grant agreements 720824 and 814650); the Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas, grant 2018-00597); and the Swedish Research Council (VR, grant 2019-04624).
Author information
Authors and Affiliations
Contributions
J.G., A.T.R., M.A. and E.J.K. wrote the code. Y.C., J.G., A.T.R., M.A., I.D., C.K., F.L., L.Y. and E.J.K. contributed to the software design. Y.C., C.K. and F.L. tested the protocol. Y.C., J.G. and E.J.K. drafted the manuscript. A.T.R., M.A., I.D., C.K., F.L., L.Y. and J.N. edited the manuscript. E.J.K. supervise the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Sriram Chandrasekaran and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Domenzain, I. et al. Nat. Commun. 13, 3766 (2022): https://doi.org/10.1038/s41467-022-31421-1
Li, F. et al. Nat. Catal. 5, 662–672 (2022): https://doi.org/10.1038/s41929-022-00798-z
Sánchez, B. J. et al. Mol. Syst. Biol. 13, 935 (2017): https://doi.org/10.15252/msb.20167411
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Gustafsson, J., Tafur Rangel, A. et al. Reconstruction, simulation and analysis of enzyme-constrained metabolic models using GECKO Toolbox 3.0. Nat Protoc 19, 629–667 (2024). https://doi.org/10.1038/s41596-023-00931-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00931-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.