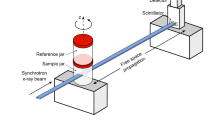
Abstract
X-ray computed tomography is a reliable technique for the detection and longitudinal monitoring of pulmonary nodules. In preclinical stages of diagnostic or therapeutic development, the miniaturized versions of the clinical computed tomography scanners are ideally suited for carrying out translationally-relevant research in conditions that closely mimic those found in the clinic. In this Protocol, we provide image acquisition parameters optimized for low radiation dose, high-resolution and high-throughput computed tomography imaging using three commercially available micro-computed tomography scanners, together with a detailed description of the image analysis tools required to identify a variety of lung tumor types, characterized by specific radiological features. For each animal, image acquisition takes 4–8 min, and data analysis typically requires 10–30 min. Researchers with basic training in animal handling, medical imaging and software analysis should be able to implement this protocol across a wide range of lung cancer models in mice for investigating the molecular mechanisms driving lung cancer development and the assessment of diagnostic and therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (https://doi.org/10.1126/scitranslmed.aaw7999 and https://doi.org/10.1038/s41467-021-26214-x). The raw datasets are too large to be publicly shared but are available for research purposes from the corresponding authors upon reasonable request. Additional data and software handling information for CT analysis are available here: https://doi.org/10.6084/m9.figshare.22355029.v2.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 71, 209–249 (2021).
Graham, M. L. & Prescott, M. J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 759, 19–29 (2015).
Hounsfield, G. N. Computed medical imaging. Nobel lecture, December 8, 1979. J. Comput. Assist. Tomogr. 4, 665–674 (1980).
Lev, M. H. & Gonzalez, R. G. in Brain Mapping: The Methods (Second Edition) (eds Arthur W. Toga & John C. Mazziotta) 427–484 (Academic Press, 2002).
Bibb, R., Eggbeer, D. & Paterson, A. in Medical Modelling (Second Edition) (eds R. Bibb, D. Eggbeer, & A. Paterson) 7–34 (Woodhead Publishing, 2015).
Jonas, D. E. et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA 325, 971–987 (2021).
Castellano, E. et al. Requirement for interaction of PI3-kinase p110α with RAS in lung tumor maintenance. Cancer Cell 24, 617–630 (2013).
de Bruin, E. C. et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 4, 606–619 (2014).
Molina-Arcas, M. et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci. Transl. Med. 11, eaaw7999 (2019).
Spiro, J. E. et al. Monitoring treatment effects in lung cancer-bearing mice: clinical CT and clinical MRI compared to micro-CT. Eur. Radiol. Exp. 4, 31 (2020).
Rudyanto, R. D. et al. Individual nodule tracking in micro-CT images of a longitudinal lung cancer mouse model. Med. Image Anal. 17, 1095–1105 (2013).
Holdsworth, D. W. & Thornton, M. M. Micro-CT in small animal and specimen imaging. Trends Biotechnol. 20, S34–S39 (2002).
Clark, D. P. & Badea, C. T. Micro-CT of rodents: state-of-the-art and future perspectives. Phys. Med. 30, 619–634 (2014).
Ford, N. L., Wheatley, A. R., Holdsworth, D. W. & Drangova, M. Optimization of a retrospective technique for respiratory-gated high speed micro-CT of free-breathing rodents. Phys. Med. Biol. 52, 5749–5769 (2007).
Ertel, D., Kyriakou, Y., Lapp, R. M. & Kalender, W. A. Respiratory phase-correlated micro-CT imaging of free-breathing rodents. Phys. Med. Biol. 54, 3837–3846 (2009).
Kumar, M. S. et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 149, 642–655 (2012).
Foster, H. et al. ATMIN is a tumor suppressor gene in lung adenocarcinoma. Cancer Res. 79, 5159–5166 (2019).
Boumelha, J. et al. An immunogenic model of KRAS-Mutant lung cancer enables evaluation of targeted therapy and immunotherapy combinations. Cancer Res. 82, 3435–3448 (2022).
Kennel, S. J. et al. High resolution computed tomography and MRI for monitoring lung tumor growth in mice undergoing radioimmunotherapy: correlation with histology. Med. Phys. 27, 1101–1107 (2000).
Fushiki, H. et al. Quantification of mouse pulmonary cancer models by microcomputed tomography imaging. Cancer Sci. 100, 1544–1549 (2009).
van Maldegem, F. et al. Characterisation of tumour microenvironment remodelling following oncogene inhibition in preclinical studies with imaging mass cytometry. Nat. Commun. 12, 5906 (2021).
Vande Velde, G. et al. Longitudinal micro-CT provides biomarkers of lung disease that can be used to assess the effect of therapy in preclinical mouse models, and reveal compensatory changes in lung volume. Dis. Model. Mech. 9, 91–98 (2016).
Marien, E., Hillen, A., Vanderhoydonc, F., Swinnen, J. V. & Vande Velde, G. Longitudinal microcomputed tomography-derived biomarkers for lung metastasis detection in a syngeneic mouse model: added value to bioluminescence imaging. Lab. Invest. 97, 24–33 (2017).
DuPage, M., Dooley, A. L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Westcott, P. M. et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature 517, 489–492 (2015).
Politi, K., Fan, P. D., Shen, R., Zakowski, M. & Varmus, H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis. Model. Mech. 3, 111–119 (2010).
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20, 1496–1510 (2006).
Bianchi, A. et al. In vivo MRI for effective non-invasive detection and follow-up of an orthotopic mouse model of lung cancer. NMR Biomed. 27, 971–979 (2014).
Krupnick, A. S. et al. Quantitative monitoring of mouse lung tumors by magnetic resonance imaging. Nat. Protoc. 7, 128–142 (2012).
Neijenhuis, L. K. A. et al. Near-infrared fluorescence tumor-targeted imaging in lung cancer: a systematic review. Life https://doi.org/10.3390/life12030446 (2022).
Imamura, T., Saitou, T. & Kawakami, R. In vivo optical imaging of cancer cell function and tumor microenvironment. Cancer Sci. 109, 912–918 (2018).
Christensen, J., Vonwil, D. & Shastri, V. P. Non-invasive in vivo imaging and quantification of tumor growth and metastasis in rats using cells expressing far-red fluorescence protein. PLoS ONE 10, e0132725 (2015).
Kocher, B. & Piwnica-Worms, D. Illuminating cancer systems with genetically engineered mouse models and coupled luciferase reporters in vivo. Cancer Discov. 3, 616–629 (2013).
Ju, H.-L. et al. Transgenic mouse model expressing P53R172H, luciferase, EGFP and KRASG12D in a single open reading frame for live imaging of tumor. Sci. Rep. 5, 8053 (2015).
Yeh, H. H. et al. Molecular imaging of active mutant L858R EGF receptor (EGFR) kinase-expressing nonsmall cell lung carcinomas using PET/CT. Proc. Natl Acad. Sci. USA 108, 1603–1608 (2011).
Price, D. N. et al. Longitudinal assessment of lung cancer progression in mice using the sodium iodide symporter reporter gene and SPECT/CT imaging. PloS ONE 11, e0169107 (2016).
Marsee, D. K. et al. Imaging of metastatic pulmonary tumors following NIS gene transfer using single photon emission computed tomography. Cancer Gene Ther. 11, 121–127 (2004).
Nielsen, C. H. et al. PET imaging of tumor neovascularization in a transgenic mouse model with a novel 64Cu-DOTA-knottin peptide. Cancer Res. 70, 9022–9030 (2010).
Umeda, I. O. et al. High resolution SPECT imaging for visualization of intratumoral heterogeneity using a SPECT/CT scanner dedicated for small animal imaging. Ann. Nucl. Med. 26, 67–76 (2012).
Khalil, M. M., Tremoleda, J. L., Bayomy, T. B. & Gsell, W. Molecular SPECT imaging: an overview. Int. J. Mol. Imaging 2011, 796025 (2011).
Hekman, M. C. H. et al. Detection of micrometastases using SPECT/fluorescence dual-modality imaging in a CEA-expressing tumor model. J. Nucl. Med. 58, 706–710 (2017).
Zhang, Y. et al. Preliminary application of micro-SPECT/CT imaging by 99mTc-tricine-EDDA-HYNIC-c-Met for non-small-cell lung cancer. Chem. Biol. Drug Des. 93, 447–453 (2019).
Versagli, C. et al. Multimodal optical, X-ray CT, and SPECT imaging of a mouse model of breast cancer lung metastasis. Curr. Mol. Med. https://doi.org/10.2174/1566524011313030006 (2013).
V, G. et al. Development of novel approach to diagnostic imaging of lung cancer with 18F-Nifene PET/CT using A/J mice treated with NNK. J. Cancer Res. Ther. 1, 128–137 (2013).
Puaux, A.-L. et al. A comparison of imaging techniques to monitor tumor growth and cancer progression in living animals. Int. J. Mol. Imaging 2011, 321538 (2011).
Yang, Z. et al. Dynamic FDG-PET imaging to differentiate malignancies from inflammation in subcutaneous and in situ mouse model for non-small cell lung carcinoma (NSCLC. PLoS ONE 10, e0139089 (2015).
Molinos, C. et al. Low-dose imaging in a new preclinical total-body PET/CT scanner. Front. Med. https://doi.org/10.3389/fmed.2019.00088 (2019).
Plathow, C. et al. Computed tomography monitoring of radiation-induced lung fibrosis in mice. Investig. Radiol. 39, 600–609 (2004).
Berghen, N. et al. Radiosafe micro-computed tomography for longitudinal evaluation of murine disease models. Sci. Rep. 9, 17598 (2019).
Detombe, S. A., Dunmore-Buyze, J., Petrov, I. E. & Drangova, M. X-ray dose delivered during a longitudinal micro-CT study has no adverse effect on cardiac and pulmonary tissue in C57BL/6 mice. Acta Radiol. 54, 435–441 (2013).
Vande Velde, G. et al. Longitudinal in vivo microcomputed tomography of mouse lungs: no evidence for radiotoxicity. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L271–L279 (2015).
Li, J. et al. A novel functional CT contrast agent for molecular imaging of cancer. Phys. Med. Biol. 55, 4389–4397 (2010).
Gómez-López, S., Whiteman, Z. E. & Janes, S. M. Mapping lung squamous cell carcinoma pathogenesis through in vitro and in vivo models. Commun. Biol. 4, 937 (2021).
You, M. S., Rouggly, L. C., You, M. & Wang, Y. Mouse models of lung squamous cell carcinomas. Cancer Metastasis Rev. 32, 77–82 (2013).
Singh, A. P., Adrianzen Herrera, D., Zhang, Y., Perez-Soler, R. & Cheng, H. Mouse models in squamous cell lung cancer: impact for drug discovery. Expert Opin. Drug Discov. 13, 347–358 (2018).
Ruiz, E. J. et al. LUBAC determines chemotherapy resistance in squamous cell lung cancer. J. Exp. Med. 216, 450–465 (2019).
Montgomery, M. K. et al. Mouse lung automated segmentation tool for quantifying lung tumors after micro-computed tomography. PLoS ONE 16, e0252950 (2021).
Birk, G., Kästle, M., Tilp, C., Stierstorfer, B. & Klee, S. Automatization and improvement of μCT analysis for murine lung disease models using a deep learning approach. Respir. Res. 21, 124 (2020).
Haines, B. B. et al. A quantitative volumetric micro-computed tomography method to analyze lung tumors in genetically engineered mouse models. Neoplasia 11, 39–47 (2009).
Gallastegui, A., Cheung, J., Southard, T. & Hume, K. R. Volumetric and linear measurements of lung tumor burden from non-gated micro-CT imaging correlate with histological analysis in a genetically engineered mouse model of non-small cell lung cancer. Lab. Anim. 52, 457–469 (2018).
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 102, 1555–1577 (2010).
Jackson, E. L. et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 65, 10280–10288 (2005).
Farncombe, T. H. Software-based respiratory gating for small animal conebeam CT. Med. Phys. 35, 1785–1792 (2008).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Poludniowski, G., Landry, G., DeBlois, F., Evans, P. M. & Verhaegen, F. SpekCalc: a program to calculate photon spectra from tungsten anode x-ray tubes. Phys. Med. Biol. 54, N433–N438 (2009).
Poludniowski, G. G. & Evans, P. M. Calculation of x-ray spectra emerging from an x-ray tube. Part I. Electron penetration characteristics in x-ray targets. Med. Phys. 34, 2164–2174 (2007).
Poludniowski, G. G. Calculation of x-ray spectra emerging from an x-ray tube. Part II. X-ray production and filtration in x-ray targets. Med. Phys. 34, 2175–2186 (2007).
Meganck, J. A. & Liu, B. Dosimetry in micro-computed tomography: a review of the measurement methods, impacts, and characterization of the Quantum GX imaging system. Mol. Imaging Biol. 19, 499–511 (2017).
Acknowledgements
We dedicate this work to Francois Lassailly, who was instrumental in setting up the In Vivo Imaging Facility at the Francis Crick Institute. We thank E. de Bruin (AstraZeneca) for providing images from EGFR mutation model. We thank N. Corps (Skyscan, Bruker), S. Belenkov and J. Sharkey (PerkinElmer) and M. Kovacs (Mediso) for providing technical assistance with the respective scanners and software. We thank the Francis Crick Institute Biological Research facilities for technical assistance. This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001039), the UK Medical Research Council (FC001039) and the Wellcome Trust (FC001039).
Author information
Authors and Affiliations
Contributions
M.Z.T. developed and tested the protocol in the PET/CT scanner. M.Z.T., C.M. and T.S. developed and tested the protocol in two micro-CT scanners. M.Z.T., C.M. and T.S. acquired and analyzed the data. M.Z.T. wrote the manuscript, and C.M. and T.S. provided technical details. T.K., A.B. and J.D. supervised the study. All authors edited the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
J.D. has acted as a consultant for AstraZeneca, Jubilant, Theras, BridgeBio and Vividion, and has funded research agreements with BMS and Revolution Medicines. None of the other authors of this manuscript has a financial interest related to this work.
Peer review
Peer review information
Nature Protocols thanks Christian Dullin and Shi-Yang Pan for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Castellano, E. et al. Cancer Cell 24, 617–630 (2013): https://doi.org/10.1016/j.ccr.2013.09.012
Molina-Arcas, M. et al. Sci Transl Med 11, eaaw7999 (2019): https://doi.org/10.1126/scitranslmed.aaw7999
van Maldegem, F. et al. Nat. Commun. 12, 5906 (2021): https://doi.org/10.1038/s41467-021-26214-x
Extended data
Extended Data Fig. 1 Highlighting and binary thresholding of individual lung tumors using Analyze software.
a–e, Screenshots showing how to draw ROI around the tumour using the tool called ‘Draw wall’ (a) and adjust the binary threshold values to display the tumour area as white pixels with a black outline (b) as presented in 3D volume rendered (c), sagittal (d) and coronal images (e).
Extended Data Fig. 2 Isolation and extraction of individual lung tumours using Analyze software.
a–c, Images from 3D volume rendered (a), sagittal (b) and coronal panels (c) showing the tumour attached to the background. d–h, Screenshots showing how to use the tool called ‘Object Separator’ (d) and erase unwanted highlighted areas manually to isolate tumour nodule from the background (e) as presented in volume rendered (f), sagittal (g) and coronal images (h).
Extended Data Fig. 3 Binary thresholding and highlighting of trachea and heart using Analyze software.
a–d, Images from 3D volume rendered (a), sagittal (b) and coronal (c) panels showing part of binary thresholded trachea after adjusting the threshold values (d). e, Screenshot showing how to highlight two regions of the heart by marking and scrolling through the frames with the tool called ‘Draw’.
Extended Data Fig. 4 Calculating signal intensity of trachea, heart and air for automatic lung segmentation with Analyze software.
a, Calculation of the mean signal intensity of trachea and heart by setting parameters. b–d, Images showing step-by-step instructions to calculate signal intensity of air inside the lung starting with creating a calibration curve with results from the trachea and the heart (b), loading Image algebra from selected lung scan (c) and then dragging image into Input picture (arrow) followed by filling equation from the calibration curve for Output and naming output image with underscore (_) at the end (d). e, Image showing how to segment the lung from the background by setting threshold in region grow.
Extended Data Fig. 5 Troubleshooting of the 3D lung volume rendering using CTAn software.
a–e, Unrepresentative structures in 3D lung volume rendering (a) can be avoided by removing fat tissue (circle) (b), gas shadow from stomach (circle) (c), motion artifacts from the ribs (black arrow) (d) and the spine (black arrow) (e) from the ROI of lung.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaw Thin, M., Moore, C., Snoeks, T. et al. Micro-CT acquisition and image processing to track and characterize pulmonary nodules in mice. Nat Protoc 18, 990–1015 (2023). https://doi.org/10.1038/s41596-022-00769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00769-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.