Abstract
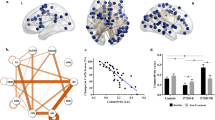
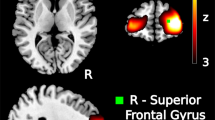
Neuromodulation trials for the treatment of posttraumatic stress disorder (PTSD) have yielded mixed results, and the optimal neuroanatomical target remains unclear. Here we analyzed three datasets to study brain circuitry causally linked to PTSD in military veterans. In veterans with penetrating traumatic brain injury, lesion locations that reduced probability of PTSD were preferentially connected to a circuit including the medial prefrontal cortex, amygdala and anterolateral temporal lobe. In veterans without lesions, PTSD was specifically associated with increased connectivity within this circuit. Reduced functional connectivity within this circuit after transcranial magnetic stimulation correlated with symptom reduction, even though the circuit was not directly targeted. This lesion-based ‘PTSD circuit’ may serve as a target for clinical trials of neuromodulation in veterans with PTSD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The functional connectivity data employed in this study are available online through the Harvard Dataverse at https://doi.org/10.7910/DVN/ILXIKS. Individual patient data cannot be shared publicly because imaging and psychiatric scales may contain identifiable information. Multiple datasets from multiple institutions are reported in this paper; data are available upon reasonable request with an approved data use agreement with the institution at which each dataset was collected. Data sharing will be subject to the policies and procedures of the institution where each dataset was collected. Questions regarding the Vietnam Head Injury Study can be directed to Dr. Jordan Grafman (jgrafman@northwestern.edu). The final PTSD network map is available on NeuroVault (https://neurovault.org/images/886976/) and on our website (http://siddiqi.bwh.harvard.edu/data-code).
Code availability
The pipeline used to prepare the functional connectivity data is available at https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016. Statistical analyses were performed in MATLAB R2021b. Custom MATLAB scripts for spatial permutation testing are available on our website (https://siddiqi.bwh.harvard.edu/data-code/) and via Zenodo at https://doi.org/10.5281/zenodo.12668537 (ref. 72).
References
Xue, C. et al. A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS ONE 10, e0120270 (2015).
Sareen, J. et al. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom. Med. 69, 242–248 (2007).
Hoskins, M. et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br. J. Psychiatry 206, 93–100 (2015).
Carpenter, J. K. et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress. Anxiety 35, 502–514 (2018).
Philip, N. S. et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am. J. Psychiatry 176, 939–948 (2019).
Siddiqi, S. H., Kording, K. P., Parvizi, J. & Fox, M. D. Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375 (2022).
Bijanki, K. R. et al. Case series: unilateral amygdala ablation ameliorates post-traumatic stress disorder symptoms and biomarkers. Neurosurgery 87, 796–802 (2020).
Gill, J. L. et al. A pilot study of closed-loop neuromodulation for treatment-resistant post-traumatic stress disorder. Nat. Commun. 14, 2997 (2023).
Koenigs, M. et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci. 11, 232–237 (2008).
Hamani, C. et al. Patient with posttraumatic stress disorder successfully treated with deep brain stimulation of the medial prefrontal cortex and uncinate fasciculus. Biol. Psychiatry 88, e57–e59 (2020).
Hamani, C. et al. Deep brain stimulation of the subgenual cingulum and uncinate fasciculus for the treatment of posttraumatic stress disorder. Sci. Adv. 8, eadc9970 (2022).
Cirillo, P. et al. Transcranial magnetic stimulation in anxiety and trauma-related disorders: a systematic review and meta-analysis. Brain Behav. 9, e01284 (2019).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Yesavage, J. A. et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US Veterans: a randomized clinical trial. JAMA Psychiatry 75, 884–893 (2018).
Isserles, M. et al. Deep transcranial magnetic stimulation combined with brief exposure for post-traumatic stress disorder: a prospective multisite randomized trial. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2021.04.019 (2021).
Reznikov, R., Binko, M., Nobrega, J. N. & Hamani, C. Deep brain stimulation in animal models of fear, anxiety, and posttraumatic stress disorder. Neuropsychopharmacology 41, 2810–2817 (2016).
George, M. S. et al. Novel treatments of mood disorders based on brain circuitry (ECT, MST, TMS, VNS, DBS). Semin. Clin. Neuropsychiatry 7, 293–304 (2002).
Siddiqi, S. H. et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat. Hum. Behav. 5, 1707–1716 (2021).
Raymont, V., Salazar, A. M., Krueger, F. & Grafman, J. “Studying injured minds” – the Vietnam Head Injury Study and 40 years of brain injury research. Front. Neurol. 2, 15 (2011).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Maller, J. J. et al. Revealing the hippocampal connectome through super-resolution 1150-direction diffusion MRI. Sci. Rep. 9, 2418 (2019).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Fan, L. et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
Bates, E. et al. Voxel-based lesion–symptom mapping. Nat. Neurosci. 6, 448–450 (2003).
Joutsa, J. et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat. Med. 28, 1249–1255 (2022).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665 (2011).
Shehzad, Z. et al. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 93, 74–94 (2014).
Siddiqi, S. H. et al. Precision functional MRI mapping reveals distinct connectivity patterns for depression associated with traumatic brain injury. Sci. Transl. Med. 15, eabn0441 (2023).
Siddiqi, S. H. et al. Repetitive transcranial magnetic stimulation with resting-state network targeting for treatment-resistant depression in traumatic brain injury: a randomized, controlled, double-blinded pilot study. J. Neurotrauma 36, 1361–1374 (2019).
Fox, M. D., Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243 (2012).
Eldaief, M. C., Halko, M. A., Buckner, R. L. & Pascual-Leone, A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl Acad. Sci. USA 108, 21229–21234 (2011).
Borgomaneri, S. et al. State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Curr. Biol. 30, 3672–3679.e3674 (2020).
Su, S. et al. Continuous theta-burst stimulation over the right dorsolateral prefrontal cortex disrupts fear memory reconsolidation in humans. iScience 25, 103614 (2022).
Notzon, S. et al. Psychophysiological effects of an iTBS modulated virtual reality challenge including participants with spider phobia. Biol. Psychol. 112, 66–76 (2015).
Herrmann, M. J. et al. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 10, 291–297 (2017).
Guhn, A. et al. Medial prefrontal cortex stimulation modulates the processing of conditioned fear. Front. Behav. Neurosci. 8, 44 (2014).
Deng, J. et al. Augmentation of fear extinction by theta-burst transcranial magnetic stimulation of the prefrontal cortex in humans. J. Psychiatry Neurosci. 46, E292–e302 (2021).
Pavlov, P. I. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 17, 136–141 (2010).
Milad, M. R. & Quirk, G. J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151 (2012).
Milad, M. R. et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454 (2007).
Schiller, D. & Delgado, M. R. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 14, 268–276 (2010).
Gouveia, F. V. et al. Neuromodulation strategies in post-traumatic stress disorder: from preclinical models to clinical applications. Brain Sci. 9, 45 (2019).
Stevens, J. S. et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 47, 1469–1478 (2013).
Morgan, M. A. & LeDoux, J. E. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 109, 681–688 (1995).
Giustino, T. F. & Maren, S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 9, 298 (2015).
Legrand, M. et al. Prefrontal cortex rTMS reverses behavioral impairments and differentially activates c-Fos in a mouse model of post-traumatic stress disorder. Brain Stimul. 12, 87–95 (2019).
Isserles, M. et al. Deep transcranial magnetic stimulation combined with brief exposure for posttraumatic stress disorder: a prospective multisite randomized trial. Biol. Psychiatry 90, 721–728 (2021).
Hartley, C. A. & Phelps, E. A. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35, 136–146 (2010).
Ochsner, K. N., Bunge, S. A., Gross, J. J. & Gabrieli, J. D. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 14, 1215–1229 (2002).
Kozel, F. A. et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 273, 153–162 (2019).
Kozel, F. A. et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: a randomized clinical trial. J. Affect. Disord. 229, 506–514 (2018).
Boggio, P. S. et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry 71, 992–999 (2010).
Leong, K. et al. A randomized sham-controlled trial of 1-Hz and 10-Hz repetitive transcranial magnetic stimulation (rTMS) of the right dorsolateral prefrontal cortex in civilian post-traumatic stress disorder: Un essai randomisé contrôlé simulé de stimulation magnétique transcrânienne repetitive (SMTr) de 1 Hz et 10 Hz du cortex préfrontal dorsolatéral droit dans le trouble de stress post-traumatique chez des civils. Can. J. Psychiatry 65, 770–778 (2020).
Cohen, H. et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry 161, 515–524 (2004).
Gordon, E. M., Laumann, T. O., Adeyemo, B. & Petersen, S. E. Individual variability of the system-level organization of the human brain. Cereb. Cortex 27, 386–399 (2017).
Hughes, K. C. & Shin, L. M. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev. Neurother. 11, 275–285 (2011).
Morey, R. A. et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch. Gen. Psychiatry 69, 1169–1178 (2012).
Belleau, E. L. et al. Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms. Neurobiol. Stress 12, 100217 (2020).
Haris, E. M., Bryant, R. A., Williamson, T. & Korgaonkar, M. S. Functional connectivity of amygdala subnuclei in PTSD: a narrative review. Mol. Psychiatry 28, 3581–3594 (2023).
Greco, J. A. & Liberzon, I. Neuroimaging of fear-associated learning. Neuropsychopharmacology 41, 320–334 (2016).
Siddiqi, S. H., Taylor, J. J., Horn, A. & Fox, M. D. Bringing human brain connectomics to clinical practice in psychiatry. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2022.05.026 (2022).
Laumann, T. O. et al. Functional system and areal organization of a highly sampled individual human brain. Neuron 87, 657–670 (2015).
Zhang, Y., Kimberg, D. Y., Coslett, H. B., Schwartz, M. F. & Wang, Z. Multivariate lesion-symptom mapping using support vector regression. Hum. Brain Mapp. 35, 5861–5876 (2014).
Siddiqi, S. H. et al. Lesion network localization of depression in multiple sclerosis. Nat. Ment. Health 1, 36–44 (2023).
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397 (2014).
Klomjai, W., Katz, R. & Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 58, 208–213 (2015).
McCalley, D. M. et al. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci. Rep. 11, 8726 (2021).
McClintock, S. M. et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry https://doi.org/10.4088/JCP.16cs10905 (2018).
Jiang, J., Ferguson, M. A., Grafman, J., Cohen, A. L. & Fox, M. D. A lesion-derived brain betwork for emotion regulation. Biol. Psychiatry 94, 640–649 (2023).
Watts, B. V., Landon, B., Groft, A. & Young-Xu, Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 5, 38–43 (2012).
Ahmadizadeh, M. J. & Rezaei, M. Unilateral right and bilateral dorsolateral prefrontal cortex transcranial magnetic stimulation in treatment post-traumatic stress disorder: a randomized controlled study. Brain Res. Bull. 140, 334–340 (2018).
Siddiqi, S. H. et al. A potential target for noninvasive neuromodulation of PTSD symptoms derived from focal brain lesions in Veterans. Zenodo https://doi.org/10.5281/zenodo.12668537 (2024).
Acknowledgements
We acknowledge the support of the 393 participants who volunteered for the studies that were analyzed in this paper. We also acknowledge discussions to conceptualize this project in collaboration with K. Johnson, G. Harper and C. Anderson of Neuronetics Inc., which led to an investigator-initiated grant for the present work. In addition, the investigators were funded by the following sources: S.H.S.: the NIH (grant nos. K23MH121657 and R21MH126271), the Brain and Behavior Research Foundation Young Investigator Grant, the Baszucki Brain Research Fund and the Department of Veterans Affairs (grant no. I01CX002293). M.D.F.: funded by the Nancy Lurie Marks Foundation, the Kaye Family Research Endowment, the Baszucki Brain Research Fund and the NIH (grant nos. R01MH113929, R21MH126271, R56AG069086, R01MH115949 and R01AG060987). N.S.P.: grant nos. I21 RX002032, I50 RX002864, U01 MH123427 and P20 GM130452.
Author information
Authors and Affiliations
Contributions
S.H.S., J.H.G. and M.D.F. designed the study. S.H.S. and S.T.P. were responsible for analysis and code development. J.H.G., R.A.M., N.S.P. and D.M.C. collected data. S.H.S., H.B. and J.B. were responsible for image processing. A.R.A. and M.A.F. were responsible for quality appraisal. S.H.S. and M.D.F. prepared the paper, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
There are no personal financial conflicts of interest related to the present results. S.H.S. is an owner of intellectual property involving the use of individualized resting-state network mapping to target TMS, which was filed in 2016, has yielded no royalties and does not cover the present work. S.H.S. is also a scientific consultant for Magnus Medical, received investigator-initiated research funding from Neuronetics (2019) and Brainsway (2022), received speaking fees from Brainsway (2021) and Otsuka (for PsychU.org, 2021), and is a shareholder in Brainsway (publicly traded) and Magnus Medical (not publicly traded). M.D.F. is a scientific consultant for Magnus Medical. M.D.F. also owns independent intellectual property involving the use of functional connectivity to target TMS for depression, which was filed in 2013, has yielded no royalties and does not cover the present work. N.S.P. received clinical trial support (through VA contracts) from Wave Neuroscience and Neurolief, serves on the scientific advisory board for Pulvinar Neuro and serves as a consultant for Motif Neurotech. The other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Desmond Oathes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Full PTSD circuit.
(a) The peak location in the PTSD circuit was the tapetum, which connects the two hippocampi to each other. Depicted here is the peak coordinate (−12, −38, 20) (pFWE=0.035 with Westfall-Young correction for multiple comparisons) at the magenta crosshairs, a peak voxel cluster in green (pFWE<0.05, detection p < 0.001, cluster size=888 mm3), overlaid on a map with uncorrected p < 0.05 which depicts the extent of the cluster into both medial temporal lobes. (b) We identified regions that were positively correlated (yellow) and negatively correlated (blue) to lesion locations that reduce probability of PTSD. To investigate whether the positive correlations (the “PTSD+ circuit”) or the negative correlations (the “PTSD- circuit”) were more relevant, we used both to predict PTSD diagnosis in a split-half analysis. PTSD risk was independently predicted by lesion overlap with the PTSD+ circuit (Pearson r = 0.18, p = 0.01), but not the PTSD- circuit (Pearson r = 0.02, p = 0.8). Permutation testing confirmed that the PTSD+ circuit was a significantly stronger predictor than the PTSD- circuit (mean difference Pearson r = 0.14, p = 0.02). Similar results were observed when excluding patients with subthreshold PTSD (Pearson r = 0.21 vs r = 0.03, p = 0.01).
Extended Data Fig. 2 PTSD circuit was independent of relevant covariates.
Nearly-identical circuits were generated when the primary analysis was repeated after (a) excluding subthreshold PTSD, (b) using a continuous measure of PTSD, (c) controlling for alcoholism risk, (d) controlling for MMSE, and (e) not controlling for BDI.
Extended Data Fig. 3 TMS-induced change in the PTSD circuit correlated with change in PTSD symptoms.
(a) PTSD was negatively associated with resting-state functional connectivity within our PTSD circuit, after controlling for TBI and depression. (b) The right DLPFC stimulation region showed marked inter-individual variability in connectivity to the PTSD circuit. 35% of participants showed positive connectivity (40% in the active group, 30% in the sham group). Thus, we hypothesized that there may also be variability in treatment-induced change. (c) In the active arm of a clinical trial, treatment-induced change in connectivity was negatively correlated with treatment-induced change in PTSD symptoms. In the sham arm, there was no relationship between PTSD improvement and connectivity change. There was a significant active-sham interaction on a linear mixed model (t = 3.32, p < 0.005). Note that data were rank-transformed in this dataset due to high influence of outliers in datasets with small sample sizes, but similar results were observed when not using a rank transform.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Figs. 1–3 and Box 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqi, S.H., Philip, N.S., Palm, S.T. et al. A potential target for noninvasive neuromodulation of PTSD symptoms derived from focal brain lesions in veterans. Nat Neurosci (2024). https://doi.org/10.1038/s41593-024-01772-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41593-024-01772-7