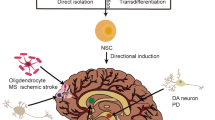
Abstract
Methods to enhance adult neurogenesis by reprogramming glial cells into neurons enable production of new neurons in the adult nervous system. Development of therapeutically viable approaches to induce new neurons is now required to bring this concept to clinical application. Here, we successfully generate new neurons in the cortex and dentate gyrus of the aged adult mouse brain by transiently suppressing polypyrimidine tract binding protein 1 using an antisense oligonucleotide delivered by a single injection into cerebral spinal fluid. Radial glial-like cells and other GFAP-expressing cells convert into new neurons that, over a 2-month period, acquire mature neuronal character in a process mimicking normal neuronal maturation. The new neurons functionally integrate into endogenous circuits and modify mouse behavior. Thus, generation of new neurons in the dentate gyrus of the aging brain can be achieved with a therapeutically feasible approach, thereby opening prospects for production of neurons to replace those lost to neurodegenerative disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data are available on request from the authors.
Code availability
No codes were used for this study.
References
D. B., D. Degeneration and regeneration of the nervous system. Nature 125, 230–231 (1930).
Bond, A. M., Ming, G. L. & Song, H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17, 385–395 (2015).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Kase, Y., Kase, Y., Shimazaki, T. & Okano, H. Current understanding of adult neurogenesis in the mammalian brain: how does adult neurogenesis decrease with age? Inflamm. Regen. 40, 10 (2020).
Mertens, J., Marchetto, M. C., Bardy, C. & Gage, F. H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424–437 (2016).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Barker, R. A., Götz, M. & Parmar, M. New approaches for brain repair—from rescue to reprogramming. Nature 557, 329–334 (2018).
Qian, H. et al. Reversing a model of Parkinson’ s disease with in situ converted nigral neurons. Nature 582, 550–556 (2020).
Zhou, H. et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell 181, 590–693 (2020).
Weinberg, M. S., Criswell, H. E., Powell, S. K., Bhatt, A. P. & McCown, T. J. Viral vector reprogramming of adult resident striatal oligodendrocytes into functional neurons. Mol. Ther. 25, 928–934 (2017).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580 (2016).
Hu, J., Qian, H., Xue, Y. & Fu, X.-D. PTB/nPTB: master regulators of neuronal fate in mammals. Biophys. Rep. 4, 204–214 (2018).
Smith, R. A. et al. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 116, 2290–2296 (2006).
Kordasiewicz, H. B. et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron 74, 1031–1044 (2012).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Miller, T. M. et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 12, 435–442 (2013).
Miller, T. et al. Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 383, 109–119 (2020).
Leavitt, B. R. & Tabrizi, S. J. Antisense oligonucleotides for neurodegeneration. Science 367, 1428–1429 (2020).
Vickers, T. A. et al. Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents. A comparative analysis. J. Biol. Chem. 278, 7108–7118 (2003).
Di Lullo, E. & Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584 (2017).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569 (2019).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Altman, J. & Das, G. D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335 (1965).
Ming, Gli & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Song, H., Berg, D. A., Bond, A. M. & Ming, Gli Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Res. 7, 277 (2018).
Song, H., Stevens, C. F. & Gage, F. H. Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44 (2002).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Morgenstern, N. A., Lombardi, G. & Schinder, A. F. Newborn granule cells in the ageing dentate gyrus. J. Physiol. 586, 3751–3757 (2008).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Heine, V. M., Maslam, S., Joëls, M. & Lucassen, P. J. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol. Aging 25, 361–375 (2004).
Schreiner, B. et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep. 12, 1377–1384 (2015).
Gerdes, J. et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133, 1710–1715 (1984).
Toni, N. et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907 (2008).
Zhao, C., Teng, E. M., Summers, R. G., Ming, G. L. & Gage, F. H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11 (2006).
Deng, W., Aimone, J. B. & Gage, F. H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 (2010).
Van Praag, H. et al. Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 (2002).
Kosik, K. S. & Finch, E. A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J. Neurosci. 7, 3142–3153 (1987).
Altman, J. & Bayer, S. A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 301, 365–381 (1990).
Kempermann, G., Song, H. & Gage, F. H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7, 9 (2015).
Wang, L.-L., Garcia, C. S., Zhong, X., Ma, S. & Zhang, C.-L. Rapid and efficient in vivo astrocyte-to-neuron conversion with regional identity and connectivity? Preprint at bioRxiv https://doi.org/10.1101/2020.08.16.253195 (2020).
Eliasson, C. et al. Intermediate filament protein partnership in astrocytes. J. Biol. Chem. 274, 23996–24006 (1999).
Van Praag, H., Kempermann, G. & Gage, F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999).
Barnes, C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104 (1979).
Heyser, C. J. & Chemero, A. Novel object exploration in mice: not all objects are created equal. Behav. Process. 89, 232–238 (2012).
Crawley, J. N. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 835, 18–26 (1999).
Gage, F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Bennett, C. F., Krainer, A. R. & Cleveland, D. W. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu. Rev. Neurosci. 42, 385–406 (2019).
Swayze, E. E. et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 35, 687–700 (2007).
Krishnakumar, R. & Blelloch, R. H. Epigenetics of cellular reprogramming. Curr. Opin. Genet. Dev. 23, 548–555 (2013).
Barca-Mayo, O. et al. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun. 8, 14336 (2017).
Clemenson, G. D. et al. Enrichment rescues contextual discrimination deficit associated with immediate shock. Hippocampus 25, 385–392 (2015).
Jafar-Nejad, P. et al. The atlas of RNase H antisense oligonucleotide distribution and activity in the CNS of rodents and non-human primates following central administration. Nucleic Acids Res. 49, 657–673 (2021).
Schafer, S. T. et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat. Neurosci. 22, 243–255 (2019).
Stafman, L. L. et al. Targeting PIM kinases affects maintenance of CD133 tumor cell population in hepatoblastoma. Transl. Oncol. 12, 200–208 (2019).
McAvoy, K. M. et al. Cell-autonomous and non-cell autonomous effects of neuronal BIN1 loss in vivo. PLoS One 14, e0220125 (2019).
Shimizu, A., Kaira, K., Yasuda, M., Asao, T. & Ishikawa, O. Decreased expression of class III β-tubulin is associated with unfavourable prognosis in patients with malignant melanoma. Melanoma Res. 26, 29–34 (2016).
James, R. E. et al. Loss of galectin-3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia 64, 105–121 (2016).
Yousef, H. et al. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 6, 11959–11978 (2015).
Zhou, J. et al. Silencing of microRNA-135b inhibits invasion, migration, and stemness of CD24+CD44+ pancreatic cancer stem cells through JADE-1-dependent AKT/mTOR pathway. Cancer Cell Int. 20, 134 (2020).
Gerdes, J. et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133, 1710–1715 (1984).
Zhao, X. & van Praag, H. Steps towards standardized quantification of adult neurogenesis. Nat. Commun. 11, 4275 (2020).
SHOLL, D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 87, 387–406 (1953).
Ting, J. T. et al. Preparation of acute brain slices using an optimized N-methyl-d-glucamine protective recovery method. J. Vis. Exp. https://doi.org/10.3791/53825 (2018).
Platzer, D. & Zorn-Pauly, K. Letter to the editor: Accurate cell capacitance determination from a single voltage step: a reminder to avoid unnecessary pitfalls. Am. J. Physiol. Heart Circ. Physiol. 311, H1072–H1073 (2016).
Mumby, D. G., Tremblay, A., Lecluse, V. & Lehmann, H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus 15, 1050–1056 (2005).
Jiang, J. et al. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90, 535–550 (2016).
Acknowledgements
This work was supported by a grant from the Nomis Foundation to D.W.C., by grant no. NS27036 from the N.I.H. to D.W.C. and S.D.C., by a Veteran’s Administration grant to T.S.H, and by grants no. MH109885, no. MH100175, no. MH108528 and no. NS105969 from the NIH to A.R.M. Some of the microscopy utilized the UCSD Microscopy Core, supported by NIH grant NS047101. D.W.C. receives salary support from the Ludwig Institute for Cancer Research. R.M. is the recipient of a postdoctoral fellowship from the Hereditary Disease Foundation. We also thank A. Roberts and the mouse phenotyping facility of the Scripps Research Institute for performing the behavioral tests in this manuscript.
Author information
Authors and Affiliations
Contributions
R.M., C.C.-M., S.D.C. and D.W.C. conceived the study. R.M., C.C.-M., C.E.S., S.M.S., K.L., F.R., C.F.B., S.D.C., T.S.H., A.R.M. and D.W.C. designed the study. R.M., C.C.-M., C.E.S., S.M.S., M.M.-D. and K.L. performed the experiments. R.M., C.C.-M. and S.M.S. analyzed the data. R.M., C.C.-M., C.E.S., S.M.S., F.R., C.F.B., S.D.C., T.S.H., A.R.M. and D.W.C. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.F.B., F.R. and K.L. are employees of, and D.W.C. is a consultant for, Ionis Pharmaceuticals. A.R.M. is a cofounder and has an equity interest in TISMOO, a company dedicated to genetic analysis and brain organoid modeling focusing on therapeutic applications customized for autism spectrum disorder and other neurological disorders with genetic origins. The terms of this arrangement have been reviewed and approved by the University of California San Diego by its conflict of interest policies.
Additional information
Peer review information Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
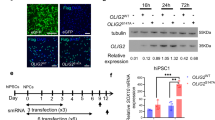
Extended Data Fig. 1 PTB and neuronal PTB (nPTB) mRNA levels 3 days post-ICV injection of PTB-ASO2.
a, PTB mRNA levels in the hippocampus, motor cortex, and striatum measured by qPCR, 3 days post ICV injection of 500 μg PTB or control ASOs into 3-months-old mouse brain. Data are presented as fold change mean +/− s.e.m. (Hippocampus: control mean: 1 +/− 0.01; PTB-ASO mean: 0.5 +/− 0.07; n = 3,n = 2 biological repeats; respectively; Motor cortex: control mean: 1 +/− 0.09; PTB-ASO mean: 0.65 +/− 0.05; n = 3 biological repeats; Striatum: control mean: 1 +/− 0.03; PTB-ASO mean: 0.86 +/− 0.04; n = 3 biological repeats). b, PTB mRNA levels in the hippocampus measured by qPCR 3 days and 15 days post ICV injection of 500 mg PTB or control ASOs into 3-month-old mouse brain. Data are presented as fold change mean +/− s.e.m. (control mean: 1 +/− 0.04; 3 days post PTB-ASOs mean: 0.61 +/− 0.08; 15 days post PTB-ASOs mean: 1.18 +/− 0.24; n = 3 biological repeats. c, Neuronal PTB (nPTB) mRNA levels in the hippocampus measured by qPCR 3 days and 15 days post ICV injection of 500 mg PTB or control ASOs into 3-month-old mouse brain. Data are presented as fold change mean +/− s.e.m. (control mean: 1 +/− 0.03; 3 days post PTB-ASOs mean: 0.63 +/− 0.04; 15 days post PTB-ASOs mean: 1.75 +/− 0.33; n = 3 biological repeats).
Extended Data Fig. 2 ASOs efficiently penetrate into and regulate gene expression in 3D human organoids in culture.
a, Representative immunofluorescence images from a 5-month-old human organoid taken 1-week post Cy3-Malat1-ASO addition to the culture medium. Images show a view of (red) Cy3-Malat1-ASO (visualized by direct immunofluorescence) or (blue) DAPI staining for DNA; experiment was reproduced two times, independently, with similar results. b, Malat1 mRNA levels measured by qPCR 2 or 4 weeks after addition to human organoid cultures or either 10 μM Malat1-ASO or control, non-targeting ASO. Data are presented as fold change mean +/− s.e.m. (2 weeks control mean: 1 +/− 0.04; 2 weeks post Malat1-ASOs mean: 0.11 +/− 0.03; 4 weeks control mean: 1 +/− 0.1; 4 weeks post Malat1-ASOs mean: 0.1 +/− 0.02; n = 3 biological repeats). c, Representative immunofluorescence images from 5-month-old human organoids taken 1-month post-Malat1-ASO application to the organoid culture medium. Images show a view of (green) a Malat1-ASO (visualized by immunofluorescence) or (blue) DAPI staining for DNA; experiment was reproduced three times, independently, with similar results.
Extended Data Fig. 3 PTB-ASO application into human organoid cultures leads to increase levels of TuJ1 protein with no alterations in SOX2 or caspase 3 markers.
a, Representative images of 5 months old human organoid taken 1-month post-PTB-ASO2 or control treatment. Images show a view of the axonal marker (green) TUJ1 (visualized by immunofluorescence); DAPI staining for DNA; experiment was reproduced three times, independently, with similar results. b, Total TuJ1 area in PTB-ASO treated organoids compared to control ASO. Data are presented as fold change mean +/− SEM (control mean: 1 +/− 0.21; PTB-ASO mean: 1.73 +/− 0.33; n = 3 biological repeats). c, Representative images and from 5 months old human organoid taken 1-month post-PTB-ASO2 application to the culture medium. Images show a view of (green) SOX2 (visualized by immunofluorescence); experiment was reproduced three times, independently, with similar results. d, Quantification of the total SOX2 positive cells per organoid area treated with PTB-ASO compared to ASO control. Data are presented as mean +/− s.e.m. (control mean: 436.7 +/− 17.82; PTB-ASO mean: 406.3 +/− 19.89; n = 3 biological repeats). e, Total caspase 3 (CC3) protein area in PTB-ASO treated organoids compared to control ASO. Data are presented as fold change mean +/− s.e.m. (control mean: 1 +/− 0.21; PTB-ASO mean: 0.79 +/− 0.19; n = 4 biological repeats).
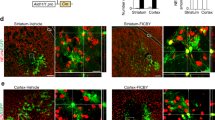
Extended Data Fig. 4 Morphology of newly generated neurons in different brain regions, 2 months post-PTB-ASO ICV administration.
Representative images of (left) granule cell layer, (middle) CA1, and (right) cortex, 2 months post-ICV injection of PTB-ASO2 into mice carrying both a CAG-lox-stop-lox-tdTomato gene and a tamoxifen-inducible GFAP-CreERT2 transgene. (Red) tdTomato (visualized by direct fluorescence); (blue) DAPI staining for DNA; experiment was reproduced three times, independently, with similar results.
Extended Data Fig. 5 New neurons along the dentate gyrus were not detected in 1.2 years old control injected mice.
a, Representative image from (left) 5 months and (right) 1.2 years old mouse dentate gyrus taken 2 months post-ICV injection of control-ASO into mice carrying both a CAG-lox-stop-lox-tdTomato gene and a tamoxifen-inducible GFAP-CreERT2 transgene. (Green) NeuN and (red) tdTomato (visualized by immunofluorescence and direct fluorescence, respectively); b, Quantification of the tdTomato + & NeuN+ neurons (iNeurons) in the granule cell layer 2 months post ICV injection of control-ASO at 5 months or 1.2 years old mice. Data are presented as fold change mean +/− s.e.m. (control 5 months mean: 1 +/− 0.35; Control 1.2 years old mean: 0 +/− 0; n = 8, n = 5 brain slides per each condition, n = 3, n = 1, respectively).
Extended Data Fig. 6 Injection of ASO to suppress PTB into the cerebral spinal fluid induces a low number of new neurons in the aged mouse cortex.
a,b, Representative image (a) from 1.2 year old mouse cortex taken 2 months post-ICV injection of PTB-ASO2 into mice carrying both a CAG-lox-stop-lox-tdTomato gene and a tamoxifen-inducible GFAP-CreERT2 transgene. b, high-magnification views of newly induced neurons (iNeurons) in the cortex expressing (green) NeuN and (red) tdTomato (visualized by immunofluorescence and direct fluorescence, respectively); (blue) DAPI staining for DNA; experiment was reproduced three times, independently, with similar results.
Extended Data Fig. 7 ICV delivery of PTB-ASO facilitate Ki67 expression in the mouse dentate gyrus and in human brain organoids.
a,b, Representative images from (a) 5 months and (b) 1.2 years old mouse dentate gyrus taken 2 months post-ICV injection of control ASO or PTB-ASO2. (Red) Ki67 (visualized by immunofluorescence); (blue) DAPI staining for DNA; experiment was reproduced three times, independently, with similar results. c, Total Ki67 positive cells per dentate gyrus of a 5 months old mouse 2 months post ICV delivery of PTB-ASO or control ASO. Data are presented as mean +/− SEM (control: 1.25 +/− 0.75; PTB-ASO mean: 5.75 +/− 2.3; n = 4 biological repeats). d, Ki67 mRNA levels measured by qPCR, 1 month after the addition of either PTB-ASO2 or control ASO to the human organoid culture medium. Data are presented as fold change mean +/− s.e.m. (control: 1 +/− 0.35; PTB-ASO mean: 4.193 +/− 1.19; n = 3 biological repeats).
Extended Data Fig. 8 DCX expression levels 2 weeks post-PTB-ASO ICV delivery into 1.5 years old mice.
Four representative images and insets of 1.5 years old mice dentate gyrus at 2 weeks post ICV delivery of (upper) control or (lower) PTB-ASO. (Green) DCX (visualized by immunofluorescence); (blue) DAPI stain for DNA; experiment was reproduced three times, independently, with similar results.
Extended Data Fig. 9 DCX protein expression levels 1 month post PTB-ASO treatment in human organoid cultures.
a, Representative images of a 5-month-old human organoid taken a 1-month post PTB or control ASO treatment. (Green) DCX visualized by immunofluorescence; (blue) DAPI to stain DNA; experiment was reproduced four times, independently, with similar results. b, Total DCX area in either PTB-ASO or control treated organoids. Data are presented as fold change mean +/− s.e.m. (control: 1 +/− 0.09; PTB-ASO mean: 1.43 +/− 0.13; n = 4 biological repeats, two tailed t test *p = 0.042).
Extended Data Fig. 10 tdTomato + , DCX + cells convert to expression of NeuN within 2 months of PTB ASO injection.
Quantification of total tdTomato + , NeuN+ positive cells per dentate gyrus area of 5 months old mice 2 weeks or 2 months post PTB-ASO or control ICV injection into mice Data are presented as mean +/− s.e.m. (control: 1.33 +/− 0.66; 2 weeks PTB-ASO mean: 3.16 +/− 1.59; 2 months PTB-ASO mean: 28.88 +/− 4.87; n = 3, n = 3, n = 4, respectively; **, One way ANOVA with Tukey’s multiple comparisons, **p = 0.0013).
Supplementary information
Supplementary Video 1
PTB-ASO-dependent iNeurons generated in the mouse dentate gyrus. High-magnification Z-stacks of an area of the dentate gyrus granule cell layer, 2 months post intracerebroventricular (ICV) injection of (video 1) control or (video 2) PTB-ASO2 into 3-month-old mice carrying both a CAG-lox-stop-lox-tdTomato gene (integrated at the Rosa locus) and a tamoxifen-inducible GFAP-CreERT2 transgene. (Green) NeuN visualized by immunofluorescence; (red) tdTomato visualized by direct fluorescence; the experiment was reproduced four times, independently, with similar results.
Supplementary Video 2
PTB-ASO-dependent iNeurons generated in the mouse dentate gyrus. High-magnification Z-stacks of an area of the dentate gyrus granule cell layer, 2 months post intracerebroventricular (ICV) injection of (video 1) control or (video 2) PTB-ASO2 into 3-month-old mice carrying both a CAG-lox-stop-lox-tdTomato gene (integrated at the Rosa locus) and a tamoxifen-inducible GFAP-CreERT2 transgene. (Green) NeuN visualized by immunofluorescence; (red) tdTomato visualized by direct fluorescence; the experiment was reproduced four times, independently, with similar results.
Supplementary Video 3
Unbiased quantification method to measure the number of total new neurons and total GFAP-positive cell number in the mouse dentate gyrus. Representative image of the output mask image created by the algorithm used to quantify the number of total tdTomato+, NeuN+ cells and the number of GFAP+ cells in the dentate gyrus area 2 months post PTB-ASO injection.
Supplementary Video 4
PTB-ASO-dependent iNeurons expressing MAP2, a marker of mature dendrites. High-magnification Z-stacks of an area of the dentate gyrus granule cell layer, 2 months post intracerebroventricular (ICV) injection of PTB-ASO2 into 3-month-old mice carrying both a CAG-lox-stop-lox-tdTomato gene (integrated at the Rosa locus) and a tamoxifen-inducible GFAP-CreERT2 transgene. (Green) MAP2 visualized by immunofluorescence; (red) tdTomato visualized by direct fluorescence; the experiment was reproduced three times, independently, with similar results.
Supplementary Video 5
PTB-ASO-dependent immature neurons expressing DCX do not colocalize with GFAP marker. High-magnification 3D video of an area of the dentate gyrus granule cell layer 2 weeks post intracerebroventricular (ICV) injection of PTB-ASO2 into 3-month-old mice. (Green) GFAP; (red) DCX (both visualized by immunofluorescence); the experiment was reproduced three times, independently, with similar results.
Supplementary Video 6
Radial glial-like cells exhibit hybrid cell markers and morphology 1 week post PTB-ASO delivery. 1 week post (ICV) injection of PTB-ASO2 into 3-month-old mice carrying both a CAG-lox-stop-lox-tdTomato gene (integrated at the Rosa locus) and a tamoxifen-inducible GFAP-CreERT2 transgene. (Green) GFAP; (magenta) DCX (both visualized by immunofluorescence). (Red) tdTomato visualized by direct fluorescence; the experiment was reproduced three times, independently, with similar results.
Rights and permissions
About this article
Cite this article
Maimon, R., Chillon-Marinas, C., Snethlage, C.E. et al. Therapeutically viable generation of neurons with antisense oligonucleotide suppression of PTB. Nat Neurosci 24, 1089–1099 (2021). https://doi.org/10.1038/s41593-021-00864-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00864-y
This article is cited by
-
Ptbp1 knockdown failed to induce astrocytes to neurons in vivo
Gene Therapy (2023)
-
The promise of microRNA-based therapies in Alzheimer’s disease: challenges and perspectives
Molecular Neurodegeneration (2021)
-
Generating neurons in the adult brain
Nature Reviews Drug Discovery (2021)