Abstract
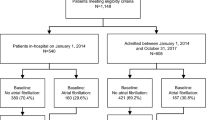
The role of antithrombotic therapy in the prevention of ischemic stroke after non-cardiac surgery is unclear. In this study, we tested the hypothesis that the association of new-onset postoperative atrial fibrillation (POAF) on ischemic stroke can be mitigated by postoperative oral anticoagulation therapy. Of 251,837 adult patients (155,111 female (61.6%) and 96,726 male (38.4%)) who underwent non-cardiac surgical procedures at two sites, POAF was detected in 4,538 (1.8%) patients. The occurrence of POAF was associated with increased 1-year ischemic stroke risk (3.6% versus 2.3%; adjusted risk ratio (RRadj) = 1.60 (95% confidence interval (CI): 1.37–1.87), P < 0.001). In patients with POAF, the risk of developing stroke attributable to POAF was 1.81 (95% CI: 1.44–2.28; P < 0.001) without oral anticoagulation, whereas, in patients treated with anticoagulation, no significant association was observed between POAF and stroke (RRadj = 1.04 (95% CI: 0.71–1.51), P = 0.847, P for interaction = 0.013). Furthermore, we derived and validated a computational model for the prediction of POAF after non-cardiac surgery based on demographics, comorbidities and procedural risk. These findings suggest that POAF is predictable and associated with an increased risk of postoperative ischemic stroke in patients who do not receive postoperative anticoagulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Access to anonymized patient data is subject to a data-sharing agreement and protocol approval from the institutional review board committee. The study-specific analyzable dataset is, therefore, not publicly available. Due to the sensitive nature of the patient data collected for this study, requests to access the dataset may be directed to the corresponding author, M.E., at meikermann@montefiore.org (14-d response time).
Code availability
Codes for inclusion criteria, baseline characteristics and outcome events were developed based on the International Classification of Diseases code classification, Current Procedural Terminology or data from electronic health records, unless otherwise specified. These are described in the supplementary materials of this manuscript. Statistical analyses were conducted using Stata (version 17), GraphPad Prism (version 9) and G*Power (version 3.1.9.4). Detailed information about the codes and packages used in the analyses can be found in the Supplementary Information. For additional clarification of these codes, contact the corresponding author at meikermann@montefiore.org (14-d response time).
References
Gialdini, G. et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA 312, 616–622 (2014).
Butt, J. H. et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J. Am. Coll. Cardiol. 72, 2027–2036 (2018).
Bhave, P. D., Goldman, L. E., Vittinghoff, E., Maselli, J. & Auerbach, A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am. Heart J. 164, 918–924 (2012).
Day, R. W. et al. Incidence and impact of postoperative atrial fibrillation after minimally invasive esophagectomy. Dis. Esophagus 29, 583–588 (2016).
Christians, K. K., Wu, B., Quebbeman, E. J. & Brasel, K. J. Postoperative atrial fibrillation in noncardiothoracic surgical patients. Am. J. Surg. 182, 713–715 (2001).
Joshi, K. K., Tiru, M., Chin, T., Fox, M. T. & Stefan, M. S. Postoperative atrial fibrillation in patients undergoing non-cardiac non-thoracic surgery: a practical approach for the hospitalist. Hosp. Pract. (1995) 43, 235–244 (2015).
McIntyre, W. F. et al. Incidence and recurrence of new-onset atrial fibrillation detected during hospitalization for non-cardiac surgery: a systematic review and meta-analysis. Can. J. Anaesth. 68, 1045–1056 (2021).
Jokinen, J. D. V. et al. Wireless single-lead ECG monitoring to detect new-onset postoperative atrial fibrillation in patients after major noncardiac surgery: a prospective observational study. Anesth. Analg. 135, 100–109 (2022).
McIntyre, W. F. et al. Atrial fibrillation recurrence in patients with transient new-onset atrial fibrillation detected during hospitalization for noncardiac surgery or medical illness: a matched cohort study. Ann. Intern. Med. 176, 1299–1307 (2023).
Fragão-Marques, M. et al. Impact of oral anticoagulation therapy on postoperative atrial fibrillation outcomes: a systematic review and meta-analysis. Thromb. J. 19, 89 (2021).
Yao, R. J. R. et al. Anticoagulation management of postoperative atrial fibrillation after cardiac surgery: a systematic review. J. Card. Surg. 36, 2081–2094 (2021).
Neves, I. A. et al. Anticoagulation therapy in patients with post-operative atrial fibrillation: systematic review with meta-analysis. Vasc. Pharm. 142, 106929 (2022).
Elharram, M. et al. Anticoagulant use and the risk of thromboembolism and bleeding in postoperative atrial fibrillation after noncardiac surgery. Can. J. Cardiol. 37, 391–399 (2021).
Hindricks, G. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 42, 373–498 (2021).
Joglar, J. A. et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 149, e1–e156 (2024).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Meersch, M. et al. Effect of intraoperative handovers of anesthesia care on mortality, readmission, or postoperative complications among adults. JAMA 327, 2403–2412 (2022).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39 (2015).
Stronati, G. et al. Derivation and validation of a clinical score for predicting postoperative atrial fibrillation in noncardiac elective surgery (the HART score). Am. J. Cardiol. 170, 56–62 (2022).
Patel, A. Y., Eagle, K. A. & Vaishnava, P. Cardiac risk of noncardiac surgery. J. Am. Coll. Cardiol. 66, 2140–2148 (2015).
Bruins, P. et al. Activation of the complement system during and after cardiopulmonary bypass surgery. Circulation 96, 3542–3548 (1997).
Amar, D., Zhang, H., Miodownik, S. & Kadish, A. H. Competing autonomic mechanisms precedethe onset of postoperative atrial fibrillation. J. Am. Coll. Cardiol. 42, 1262–1268 (2003).
Prince-Wright, L. H. et al. Postoperative atrial fibrillation following non-cardiac surgery: predictors and risk of mortality. Am. J. Surg. 224, 1062–1067 (2022).
Lee, S.-H. et al. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am. Heart J. 167, 593–600 (2014).
Lin, M. H. et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality: a meta-analysis. Stroke 50, 1364–1371 (2019).
Huynh, J. T. et al. Association between perioperative atrial fibrillation and long-term risks of stroke and death in noncardiac surgery: systematic review and meta-analysis. CJC Open 3, 666–674 (2021).
Siontis, K. C. et al. Associations of atrial fibrillation after noncardiac surgery with stroke, subsequent arrhythmia, and death. Ann. Intern. Med. 175, 1065–1072 (2022).
Hindricks, G. et al. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 4194 (2021).
Devereaux, P. J. et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371, 1839–1847 (2008).
Conen, D. et al. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): an international randomised trial. Lancet 402, 1627–1635 (2023).
Ha, A. C. T. et al. Effect of continuous electrocardiogram monitoring on detection of undiagnosed atrial fibrillation after hospitalization for cardiac surgery. JAMA Netw. Open 4, e2121867 (2021).
El‐Chami, M. F. et al. Management of new‐onset postoperative atrial fibrillation utilizing insertable cardiac monitor technology to observe recurrence of AF (MONITOR‐AF). Pacing Clin. Electrophysiol. 39, 1083–1089 (2016).
Yao, X. et al. Artificial intelligence–enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat. Med. 27, 815–819 (2021).
Attia, Z. I. et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 25, 70–74 (2019).
Friedrich, S. et al. Patent foramen ovale and long-term risk of ischaemic stroke after surgery. Eur. Heart J. 40, 914–924 (2019).
Ng, P. Y. et al. Association of preoperatively diagnosed patent foramenovale with perioperative ischemic stroke. JAMA 319, 452–462 (2018).
Marcucci, M., Chan, M. T. V., Smith, E. E., Absalom, A. R. & Devereaux, P. J. Prevention of perioperative stroke in patients undergoing non-cardiac surgery. Lancet Neurol. 22, 946–958 (2023).
Ke Wang, M. et al. Anticoagulation use in perioperative atrial fibrillation after noncardiac surgery: a systematic review and meta-analysis. Swiss Med. Wkly 153, 40056 (2023).
Kotalczyk, A., Mazurek, M., Kalarus, Z., Potpara, T. S. & Lip, G. Y. H. Stroke prevention strategies in high-risk patients with atrial fibrillation. Nat. Rev. Cardiol. 18, 276–290 (2021).
Spyropoulos, A. C. & Douketis, J. D. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 120, 2954–2962 (2012).
Vandenbroucke, J. P. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: explanation and elaboration. PLoS Med. 4, e296 (2007).
Benchimol, E. I. et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 12, e1001885 (2015).
Schneeweiss, S. et al. Graphical depiction of longitudinal study designs in health care databases. Ann. Intern. Med. 170, 398–406 (2019).
Siontis, K. C. et al. Association of new-onset atrial fibrillation after noncardiac surgery with subsequent stroke and transient ischemic attack. JAMA 324, 871–878 (2020).
Brott, T. et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20, 864–870 (1989).
Shay, D. et al. Preoperative heart failure treatment prevents postoperative cardiac complications in patients with lower risk: a retrospective cohort study. Ann. Surg. 277, e33–e39 (2023).
Ahrens, E. et al. Prevalence and association of non-medical cannabis use with post-procedural healthcare utilisation in patients undergoing surgery or interventional procedures: a retrospective cohort study. EClinicalMedicine 57, 101831 (2023).
Scheffenbichler, F. T. et al. Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Crit. Care Med. 49, e247–e257 (2021).
Schaefer, M. S. et al. What factors predict adverse discharge disposition in patients older than 60 years undergoing lower-extremity surgery? The Adverse Discharge in Older Patients after Lower-extremity Surgery (ADELES) risk score. Clin. Orthop. Relat. Res. 479, 546–547 (2021).
Aalen, O. O. & Johansen, S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand. J. Stat. 5, 141–150 (1978).
Kurth, T. et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am. J. Epidemiol. 163, 262–270 (2006).
Webster‐Clark, M. et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat. Med. 40, 1718–1735 (2021).
Lip, G. Y. H., Nieuwlaat, R., Pisters, R., Lane, D. A. & Crijns, H. J. G. M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest 137, 263–272 (2010).
Yaggi, H. & Mohsenin, V. Obstructive sleep apnoea and stroke. Lancet Neurol. 3, 333–342 (2004).
Barnes, M. E. et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin. Proc. 79, 1008–1014 (2004).
Bouzas-Mosquera, A. et al. Left atrial size and risk for all-cause mortality and ischemic stroke. Can. Med. Assoc. J. 183, E657–E664 (2011).
Mannina, C. et al. Association of left atrial strain with ischemic stroke risk in older adults. JAMA Cardiol. 8, 317–325 (2023).
Zou, G. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Yelland, L. N., Salter, A. B. & Ryan, P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am. J. Epidemiol. 174, 984–992 (2011).
Pedroza, C. & Truong, V. T. T. Estimating relative risks in multicenter studies with a small number of centers—which methods to use? A simulation study. Trials 18, 512 (2017).
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5, 1315–1316 (2010).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Acknowledgements
The authors declare no specific grants for this research from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Consortia
Contributions
O.A., L.Z., M.S.S. and M.E. had access to the data. M.E. is the guarantor of the manuscript and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: O.A., M.K., W.J.S., M.S.S., M.G., D.L.B., L.D. and M.E. Acquisition, analysis or interpretation of the data: O.A., T.T.H., L.Z., M.D., M.I.R., F.B. and D.W. Drafting of the manuscript: O.A., M.I.R., F.Z., A.E., D.L.B. and M.E. Critical revision of the manuscript: all authors. Statistical analysis: O.A., I.M.K., M.D., M.I.R., F.B. and K.W. D.L.B. and M.E. supervised critical revisions.
Corresponding author
Ethics declarations
Competing interests
M.E. receives funding from the National Institutes of Health (NIH) (R01AG065554 and R01HL132887) that does not pertain to this manuscript. He holds two patents for acyclic curcubiturils for reversal of drugs of abuse and neuromuscular blocking agents (patent numbers 9956229 and 9469648). He is a member of the associated editorial board for the British Journal of Anaesthesia. D.L.B. discloses the following relationships. Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma and Stasys; Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options) and High Enroll (stock); Consultant: Broadview Ventures, Hims, SFJ and Youngene; Data Monitoring Committee: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (chair, PEITHO trial), Cleveland Clinic, Contego Medical (chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute and Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (ACC) (senior associate editor, Clinical Trials and News; chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee, funded by Boehringer Ingelheim; AEGIS-II executive committee, funded by CSL Behring), Belvoir Publications (editor in chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (American Heart Association lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor and associate editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (course director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee and steering committee and USA national co-leader, funded by Bayer), WebMD (CME steering committees) and Wiley (steering committee); Other: Clinical Cardiology (deputy editor); Patent: sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital, assigned to Lexicon; neither D.L.B. nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Eli Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene and 89Bio; Royalties: Elsevier (editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte and Vascular Solutions; Trustee: ACC; Unfunded Research: FlowCo. M.S.S. received funding for investigator-initiated studies from Merck & Co., which does not pertain to this manuscript. He is an associate editor for BMC Anesthesiology. He received honoraria for lectures from Fisher & Paykel Healthcare and Mindray Medical International Limited. He received an unrestricted philanthropic grant from Jeff and Judy Buzen. All other authors have no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Peer review
Peer review information
Nature Medicine thanks Behnood Bikdeli, Jeffrey Weitz, William McIntyre and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Kaplan-Meier survival estimates.
The figure illustrates the adjusted Kaplan-Meier survival estimates analysis conducted for the occurrence of stroke in patients experiencing new onset postoperative atrial fibrillation(red line) and patients who did not(blue line). The x-axis represents time after surgery in days, while the y-axis indicates the probability of being stroke-free (Hazard Ratio: 2.07 (95% CI 1.92-2.23), p < 0.001).
Extended Data Fig. 2 Risk of new-onset postoperative atrial fibrillation and stroke.
(A) New-onset atrial fibrillation (POAF) after non-cardiac surgery is associated with postoperative complications, stroke, heart failure, and myocardial infarction. (B) For patients who were prescribed oral anticoagulants, the absolute risk difference was 0.34% in all patients and 0.68% in high-risk patients (upper quintile in computational prediction model [>11 points]). There was no significant absolute risk difference in patients who were prescribed oral anticoagulants. The images used were created by the authors using Procreate for iOS17 (Savage Interactive Pty Ltd., Hobart, Australia).
Extended Data Fig. 3 Graphical description of the study design.
Graphical description of the study design, visualizing temporal anchors of exposure (new-onset postoperative atrial fibrillation), outcome/follow-up (ischemic stroke/transient ischemic attack), covariates (baseline demographics, procedure related factors, comorbidities and preexisting medication) and primary effect modifier (oral anticoagulation). The cohort entry date (CED), serving as the primary anchor date for patients entering the study analysis, was the date of the patients' index surgery (Day 0). We excluded patients with preexisting atrial fibrillation, age below 18 years, ASA physical status greater than 4 or missing data for exposure, outcome or covariates. Covariates were assessed during a window extending one year prior to the CED if not otherwise specified [-365,-1]. The time window for the primary exposure - postoperative atrial fibrillation (POAF) - was between day 0 and the end of day post-CED. Oral anticoagulation prescriptions were included between postoperative day 1 and the end of day 365. The outcome ischemic stroke was assessed between day 31 and 365 after surgery. Abbreviations: y, years; ASA, American Society of Anesthesiologists; POAF, new-onset postoperative atrial fibrillation.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Supplementary Tables 1–7 and Supplementary Notes 1 (STROBE checklist) and 2 (Statistical Analysis Plan).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azimaraghi, O., Rudolph, M.I., Wongtangman, K. et al. Role of anticoagulation therapy in modifying stroke risk associated with new-onset atrial fibrillation after non-cardiac surgery. Nat Med (2024). https://doi.org/10.1038/s41591-024-03206-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-03206-0