Abstract
Allergic skin diseases, such as atopic dermatitis, are clinically characterized by severe itching and type 2 immunity-associated hypersensitivity to widely distributed allergens, including those derived from house dust mites (HDMs). Here we found that HDMs with cysteine protease activity directly activated peptidergic nociceptors, which are neuropeptide-producing nociceptive sensory neurons that express the ion channel TRPV1 and Tac1, the gene encoding the precursor for the neuropeptide substance P. Intravital imaging and genetic approaches indicated that HDM-activated nociceptors drive the development of allergic skin inflammation by inducing the degranulation of mast cells contiguous to such nociceptors, through the release of substance P and the activation of the cationic molecule receptor MRGPRB2 on mast cells. These data indicate that, after exposure to HDM allergens, activation of TRPV1+Tac1+ nociceptor–MRGPRB2+ mast cell sensory clusters represents a key early event in the development of allergic skin reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Zheng, T., Yu, J., Oh, M. H. & Zhu, Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol. Res. 3, 67–73 (2011).
Leung, D. Y. & Bieber, T. Atopic dermatitis. Lancet 361, 151–160 (2003).
Sager, N., Feldmann, A., Schilling, G., Kreitsch, P. & Neumann, C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J. Allergy Clin. Immunol. 89, 801–810 (1992).
Langer, K., Breuer, K., Kapp, A. & Werfel, T. Staphylococcus aureus-derived enterotoxins enhance house dust mite-induced patch test reactions in atopic dermatitis. Exp. Dermatol. 16, 124–129 (2007).
Park, H. Y. et al. Staphylococcus aureus colonization in acute and chronic skin lesions of patients with atopic dermatitis. Ann. Dermatol. 25, 410–416 (2013).
Bunikowski, R. et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J. Allergy Clin. Immunol. 105, 814–819 (2000).
McFadden, J. P., Noble, W. C. & Camp, R. D. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br. J. Dermatol. 128, 631–632 (1993).
Marichal, T. et al. Guanine nucleotide exchange factor RABGEF1 regulates keratinocyte-intrinsic signaling to maintain skin homeostasis. J. Clin. Invest. 126, 4497–4515 (2016).
Cookson, W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat. Rev. Immunol. 4, 978–988 (2004).
Holgate, S. T. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 28, 248–251 (2007).
Palmer, C. N. et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38, 441–446 (2006).
O’Regan, G. M., Sandilands, A., McLean, W. H. & Irvine, A. D. Filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 122, 689–693 (2008).
Oyoshi, M. K., He, R., Kumar, L., Yoon, J. & Geha, R. S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 102, 135–226 (2009).
LaMotte, R. H., Dong, X. & Ringkamp, M. Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci. 15, 19–31 (2014).
Salomon, J. & Baran, E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 22, 223–228 (2008).
Toyoda, M. et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 147, 71–79 (2002).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Baral, P. et al. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24, 417–426 (2018).
Moriyama, S. et al. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Wallrapp, A. et al. Erratum: the neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 551, 658 (2017).
Hofmann, A. M. & Abraham, S. N. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr. Opin. Immunol. 21, 679–686 (2009).
McNeil, B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2014).
Gaudenzio, N. et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Invest. 126, 3981–3998 (2016).
Monti, G., Tonetto, P., Mostert, M. & Oggero, R. Staphylococcus aureus skin colonization in infants with atopic dermatitis. Dermatology 193, 83–87 (1996).
Ando, T. et al. Mast cells are required for full expression of allergen/SEB-induced skin inflammation. J. Invest. Dermatol. 133, 2695–2705 (2013).
Beck, L. A. et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 371, 130–139 (2014).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Lattin, J. E. et al. Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 4, 5 (2008).
Reithofer, M. & Jahn-Schmid, B. Allergens with protease activity from house dust mites. Int. J. Mol. Sci. 18, 1368 (2017).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
Locksley, R. M. Asthma and allergic inflammation. Cell 140, 777–783 (2010).
Palm, N. W., Rosenstein, R. K. & Medzhitov, R. Allergic host defences. Nature 484, 465–472 (2012).
Stewart, G. A. & Thompson, P. J. The biochemistry of common aeroallergens. Clin. Exp. Allergy 26, 1020–1044 (1996).
Cayrol, C. et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 19, 375–385 (2018).
Reber, L. L. et al. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2, e92900 (2017).
Kim, Y. S. et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887 (2014).
Lansu, K. et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 13, 529–536 (2017).
Alving, K. et al. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res. 264, 529–538 (1991).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Hagiyama, M. et al. Increased expression of cell adhesion molecule 1 by mast cells as a cause of enhanced nerve-mast cell interaction in a hapten-induced mouse model of atopic dermatitis. Br. J. Dermatol. 168, 771–778 (2013).
Pang, X., Boucher, W., Triadafilopoulos, G., Sant, G. R. & Theoharides, T. C. Mast cell and substance P-positive nerve involvement in a patient with both irritable bowel syndrome and interstitial cystitis. Urology 47, 436–438 (1996).
Suzuki, R. et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 163, 2410–2415 (1999).
Buhner, S. et al. Calcium imaging of nerve-mast cell signaling in the human intestine. Front. Physiol. 8, 971 (2017).
Green, D. P., Limjunyawong, N., Gour, N., Pundir, P. & Dong, X. A mast cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101, 412–420 (2019).
Meixiong, J. et al. Activation of mast cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50, 1163–1171 (2019).
Vocanson, M., Hennino, A., Rozieres, A., Poyet, G. & Nicolas, J. F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 64, 1699–1714 (2009).
Che, D. et al. Mivacurium induces mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell. Immunol. 332, 121–128 (2018).
Takamori, A. et al. Identification of inhibitory mechanisms in pseudo-allergy involving Mrgprb2/MRGPRX2-mediated mast cell activation. J. Allergy Clin. Immunol. 143, 1231–1235 (2019).
Acknowledgements
We thank all members of the Galli and Gaudenzio laboratories for discussions, and C. Liu for technical assistance. We thank A. Olson and the Stanford Neuroscience Microscopy Service (supported by NIH No. NS069375); this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH. We thank F. L’Faqihi (IFR30, Plateau Technique Cytometrie, Toulouse) and S. Allart (IFR30, Plateau Technique Imagerie Cellulaire, Toulouse) for technical assistance. T.M. is a Research Associate of the F.R.S.-FNRS and is supported by an ‘Incentive Grant for Scientific Research’ of the F.R.S.-FNRS (No. F.4508.18), by the FRFS-WELBIO under grant No. CR-2017 s-04, by the Acteria Foundation and by an ERC Starting Grant (No. IM-ID 801823). P.S. acknowledges support from and the Austrian Science Fund (No. P31113-B30). L.L.R. acknowledges support from the European Commission (Marie Sklodowska-Curie Individual Fellowship No. H2020-MSCA-IF-2014 656086) and the INSERM ATIP-Avenir program. This work was supported by grants from NIH (S.J.G., grant Nos. U19 AI104209, R01 AR067145 and R01 AI32494), the United States–Israel Binational Science Foundation (No. 2013263) and the Sean N. Parker Center for Allergy and Asthma Research, Stanford University (to S.J.G.); and by the Société Française de Dermatologie, the Société Française d’Allergologie, the Marie Sklodowska-Curie Individual Fellowship (H2020-MSCA-IF-2016, No. 749629), the European Research Council (ERC-2018-STG, No. 802041) and the INSERM ATIP-Avenir program (to N.G.).
Author information
Authors and Affiliations
Contributions
S.J.G. and N.G. conceived the project. All authors were involved in experimental design. N.S., L.B. and N.G. performed most experiments and compiled the data. R.S., C.B., J.M., C.P., T.M., P.S., L.L.R., N.C., X.D. and M.T. helped with experiments. All authors participated in analyzing the data and writing or editing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Ioana Visan was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 D. farinae and SEB treatment induces the development of systemic D. farinae-specific type 2 immunity and AD-like pathology.
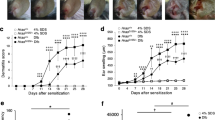
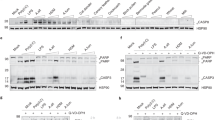
a, Protocol to induce D. farinae + SEB-mediated allergic skin inflammation. b, Clinical score calculation. c-e, WT mice were treated with SEB, D. farinae or D. farinae + SEB (or vehicle control) to induce the development allergic skin inflammation. Representative hematoxylin & eosin (H&E) staining of vehicle-, SEB-, D. farinae-, or D. farinae + SEB-treated areas. d, Clinical score (0-12) of vehicle- (n = 3 mice), SEB-(n = 5 mice), D. farinae-(n = 5 mice), or D. farinae + SEB-treated (n = 5 mice) areas from two independent experiments, mean ± SEM. e, Spleen weight measurements of vehicle- (n = 6 mice) or D. farinae + SEB-treated (n = 15 mice) mice from five independent experiments. f, Flow cytometry analysis of the proportion (%) of splenic CD4+ T lymphocytes from vehicle- (open bars) or D. farinae + SEB-treated (black bars) mice expressing intracellular IL-4, IL-5, IL-13 or IFN-γ after ex vivo restimulation with D. farinae, from vehicle- (n = 5 mice) or D. farinae + SEB-treated (n = 7 mice) mice from three independent experiments, mean + SEM; two-tailed, unpaired t-test, **P<.01 ***P<.001. g,h, WT mice were treated with D. farinae + SEB and injected intraperitoneally twice a week with an anti-mouse IL-4/13Rα blocking antibody (n=6 mice) or an isotype control (n = 6 mice), data are from two independent experiments. g, Representative H&E staining of D. farinae + SEB-treated areas. h, Clinical score (0-12), isotype control-treated vs. anti-IL-4/13Rα-treated D. farinae + SEB-treated, mean + SEM; two-tailed, unpaired t-test, *P<.05. c,g, dotted black lines indicate the junction epidermis/dermis, bars = 100 μm. Each open circle = one mouse. i, Publicly available microarray gene expression data of Trpv1, Tac1 and Trpa1 in mouse DRG and different immune cell subpopulations (GSE 10246); data are shown using a heat map of mRNA expression levels.
Supplementary Figure 2 Extended analysis of epidermal proteins in WT and Tac1-/- mice.
Representative confocal microscopy of back skin sections (a,c,e,g,i,k) and corresponding fluorescence analysis (b,d,f,h,j,l) of the indicated staining. Bars = 100 μm; dotted white lines indicate the epidermal/dermal junction. Each open circle = one mouse. Open bars: WT mice treated either with vehicle (n = 5 mice) or D. farinae + SEB (n = 14 mice); black bars: Tac1-/- mice treated with D. farinae + SEB (n = 8 mice); from three independent experiments, mean + SEM; 1-way ANOVA with Tukey’s test for multiple comparisons; *P<.05 **P<.01 ***P<.001.
Supplementary Figure 3 Extended analysis of epidermal protein organization in DMSO-treated and RTX-treated mice.
a, 4-week-old C57BL/6J WT mice were treated subcutaneously (s.c.) into the back with DMSO (Mock treated) or RTX in three escalating doses (30, 70 and 100 μg.kg-1) on consecutive days and mice were rested for 4-6 weeks before D. farinae + SEB treatment. b, Confirmation of TRPV1+ nociceptors ablation by immersion of the tail into a water bath maintained at 52oC17 and comparison of the latency to the first tail movement elicited by hot water in DMSO-treated (open bars, n = 4 mice) vs RTX-treated (black bars, n = 5 mice) group, from two independent experiments, mean + SEM; two-tailed, unpaired t-test, *P<.05. c-p, Representative confocal microscopy from two independent experiments of back skin section (c,e,g,i,k,m,o) and corresponding fluorescence analysis (d,f,h,j,l,n,p) of the indicated staining. Bars = 100 μm; dotted white lines indicate the epidermal/dermal junction. Each open circle = one mouse. All the following groups are D. farinae + SEB-treated, open bars: Mock DMSO-treated mice (n = 4 mice), black bars: RTX-treated mice (n = 5 mice); from two independent experiments mean + SEM; two-tailed, unpaired t-test, *P<.05 **P<.01.
Supplementary Figure 4 D. farinae-mediated activation of DRG neurons is not dependent on MyD88 or PAR-2 signaling and analysis of the proteolytic activity of D. farinae and/or SEB.
DRG neurons were isolated from WT mice then treated with control or MyD88 inhibitory peptide or from PAR-2-deficient mice (a,b). a, Representative Fluo-4 fields of ex vivo cultured DRG neurons from WT mice, incubated with control peptide (a, up) or MyD88 inhibitory peptide (a, mid) or from PAR-2-deficient mice (a, low) and treated with vehicle, 5 ng/ml D. farinae, or 50 mM KCL. Bars = 50 μm. b, Proportion of responding DRG neurons incubated with control peptide (white bars, n = 6 mice) or MyD88 inhibitory peptide (grey bars, n = 6 mice) or from PAR-2-deficient mice (black bars, n=8 mice) stimulated with 5 ng/ml D. farinae and expressed as % of DRG neurons responding to 50 mM KCL, each open circle = one mouse. (a,b) Data are from three independent experiments, (b) mean + SEM. c, Proteolytic activity of D. farinae extracts in presence of SEB or SEB alone and normalized to the 100% proteolytic activity obtained in the control condition D. farinae, from three independent experiments, mean + SEM.
Supplementary Figure 5 Kit-dependent and Kit-independent mast cell-deficient mice are protected from the development of a model of allergic skin inflammation.
Kit-dependent KitW-sh/W-sh mast cell-deficient mice versus Kit+/+ mast cell-sufficient littermate control mice (a-i) and Kit-independent Cpa3-cre+;Mcl-1fl/fl mast cell-deficient mice and Cpa3-cre+;Mcl-1+/+ mast cell-sufficient littermate control mice (j-r) were treated with D. farinae + SEB to induce the development allergic skin inflammation. a, Representative H&E staining of D. farinae + SEB-treated areas. b, Clinical scores (0-12), open circles: Kit+/+ mice (n = 8 mice), closed circles: KitW-sh/W-sh mice (n = 8 mice), from three independent experiments, mean ± SEM, two-tailed, unpaired t-test, ***P<.001. c, Epidermal thickness (μm). d,e, Number of eosinophils (d) and neutrophils (e) in skin sections. f-i, Representative confocal microscopy of back skin section (f,h) and fluorescence analysis (g,i) of claudin-1 (f,g) and filaggrin (h,i) stainings. Bars = 100 μm, dotted black (a) or white (f,h) lines indicate the junction epidermis/dermis. c-e,g,i, Each open circle = one mouse; white bars: Kit+/+ mice (number of mice [c-e] n = 7, [g] n = 6, [i] n = 8), black bars: KitW-sh/W-sh mice (number of mice [c-e] n = 7, [g-i] n = 8) from three independent experiments, mean ± SEM; two-tailed, unpaired t-test, *P<.05 **P<.01 ***P<.001. (j-r) same experiment as in (a-i) but in Kit-independent Cpa3-cre+;Mcl-1fl/fl mast cell-deficient mice and Cpa3-cre+;Mcl-1+/+ mast cell-sufficient littermate control mice. Each open circle = one mouse, open symbols or bars: Cpa3-cre+;Mcl-1+/+ mice (n = 4 mice), black symbols or bars: Cpa3-cre+;Mcl-1fl/fl mice (n = 4 mice); from two independent experiments mean + SEM; two-tailed, unpaired t-test, *P<.05.
Supplementary Figure 6 RTX-treated, Tac1-/- or Mrgprb2mut/mut mice are not deficient in skin mast cell.
a,b, Representative Toluidine blue (TB) staining (a) and associated mast cell counts (b) of D. farinae + SEB-treated area in Kit+/+ (n = 8 mice) and KitW-sh/W-sh mice (n = 7 mice), from three independent experiments. c,d, Same experiment in Cpa3-cre+;Mcl-1+/+ mice (n = 5 mice) and Cpa3-cre+;Mcl-1fl/fl mice (n = 5 mice), from two independent experiments. b,d, mean + SEM; two-tailed, unpaired t-test, *P<.05 ***P<.001. e,f, Same experiment but in vehicle-treated WT mice (open bar, n = 5 mice) or D. farinae + SEB-treated WT (open bars, n=7 mice, from three independent experiments), DMSO-treated (light grey bars n = 4 mice, from two independent experiments), RTX-treated (dark grey bars n = 5 mice, two independent experiments) and Tac1-/- mice (black bars, n = 7 mice, from three independent experiments). g,h, Same experiment but in untreated or D. farinae + SEB-treated in Mrgprb2+/+ mice (n = 6 mice) and Mrgprb2mu/mutt mice (n = 7 mice) (f,h). Mean + SEM; two-tailed, 1-way ANOVA with Tukey’s test for multiple comparisons. Each open circle = one mouse.
Supplementary Figure 7 Extended analysis of epidermal protein organization in Mrgprb2+/+ and Mrgprb2mut/mut mice.
Representative confocal microscopy, of back skin section (a,c,e,g,i,k) and corresponding protein fluorescence analysis (b,d,f,h,j,l) of the indicated staining. Bars = 100 μm; dotted white lines indicate the junction epidermis/dermis. Each open circle = one mouse. All the following groups are D. farinae + SEB-treated, white bars: Mrgprb2+/+ mice ([b,h,j] n = 6, [d] n = 3, [f,l] n = 6 mice), black bars: Mrgprb2mut/mut mice ([b,f,h,j,l] n = 6, [d] n = 3 mice); all from three independent experiments, mean + SEM; two-tailed, unpaired t-test, *P<.05 **P<.01 ***P<.001.
Supplementary Figure 8 Imaging methods used ex vivo and in vivo, pretreatment with E64 prevents ear swelling and AD antigens do not activate mast cells in vitro.
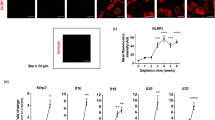
a, Protocol to analyze release of mast cell granules ex vivo in whole-mounted ear skin (related to Figure 5). 8 µg of sulforhodamine 101-labeled avidin (Av.SRho) in 20 μl PBS were intradermally (i.d.) injected into the ear pinna of WT, Mrgprb2mut/mut, or RTX-treated mice and their respective control mice to selectively label mast cell secretory granules in vivo37. 1 week later, ear thickness was measured and right ear pinnae were injected i.d. with 1 µg D. farinae + 50 ng SEB or 1 µM Capsaicin. In some experiments, right ear pinnae were injected i.d. with 1 µg D. farinae + 50 ng SEB with 15 µg of IgG anti-SP. Left ear pinnae were injected with control solutions: vehicle alone or vehicle in combination with IgG isotype. 45 minutes later, ear thickness was measured again and mice were euthanized before ear excision. 3-D images of untouched whole-mounted ears were acquired using volumetric confocal microscopy. b, 1 µg D. farinae + 50 ng SEB alone or pretreated with cysteine protease inhibitor E64 for 30 minutes at 37°C were injected respectively into the left and right ear pinna of each WT mouse (so that each mouse is its own control) in 20 μl PBS. 45 minutes later, ear thickness was measured. c, Changes (Δ) in ear thickness over time after i.d. injection of stimuli, untreated, white circles n = 10 mice and E64-pretreated, n = 10 mice, from two independent experiments, mean ± SEM, two-tailed, paired t-test, *P<.05. Each open circle = one mouse. d, β-hexosaminidase release from human mast cells (hMC) upon treatment with increasing concentrations of D. farinae and SEB, alone or together. For positive control, hMCs were sensitized with human IgE and then stimulated with 10 ng/mL anti-IgE. n = 6 independent experiments with three independent donors, mean + SEM, one-way ANOVA with Tukey’s test for multiple comparisons, ***P<.001. e, β-hexosaminidase release from mouse BMCMCs upon treatment with increasing concentrations of D. farinae and SEB, alone or together. For positive control, BMCMCs were sensitized with murine anti-DNP IgE and then challenged with 10 ng/mL DNP-BSA, n = 8 independent experiments, with BMCMCs from four mice, mean + SEM, one-way ANOVA with Tukey’s test for multiple comparisons ***P<.001. f, Protocol to analyze sensory neuron calcium levels and mast cell granules release simultaneously in living mice. 8 μg of Av.SRho in 20 μl of PBS were injected i.d. into the ear pinna of Pirt-GCaMP3 mice. 1 week later, mice were injected i.d. with vehicle, 1 μM capsaicin, 1 μg D. farinae and 50 ng SEB (used alone or in combination) in a final volume of 20 μl then placed under the two-photon microscope. 3-D images from three independent experiments were acquired and fluorescence corresponding to Av.SRho+ mast cell granule structures or GCaMP3+ neuron calcium levels were analyzed using ImageJ and/or Imaris Bitplane software. g, Examples of mean fluorescence intensity (MFI) detected in vehicle (left) and capsaicin-activated (i.e., with non-degranulated mast cells, right) sensory neurons. h, Examples of Av.SRho+ mast cells without (left) or with (i.e., degranulated mast cells, right) exteriorized mast cell granule structures.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8.
Rights and permissions
About this article
Cite this article
Serhan, N., Basso, L., Sibilano, R. et al. House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation. Nat Immunol 20, 1435–1443 (2019). https://doi.org/10.1038/s41590-019-0493-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0493-z
This article is cited by
-
IL-4Rα signalling in B cells and T cells play differential roles in acute and chronic atopic dermatitis
Scientific Reports (2023)
-
New perspectives on the origins and heterogeneity of mast cells
Nature Reviews Immunology (2023)
-
Lung-specific MCEMP1 functions as an adaptor for KIT to promote SCF-mediated mast cell proliferation
Nature Communications (2023)
-
Anatomical differences in nociceptor neurons sensitivity
Bioelectronic Medicine (2022)
-
Somatosensory and autonomic neuronal regulation of the immune response
Nature Reviews Neuroscience (2022)