Abstract
The global retreat of glaciers is dramatically altering mountain and high-latitude landscapes, with new ecosystems developing from apparently barren substrates1,2,3,4. The study of these emerging ecosystems is critical to understanding how climate change interacts with microhabitat and biotic communities and determines the future of ice-free terrains1,5. Here, using a comprehensive characterization of ecosystems (soil properties, microclimate, productivity and biodiversity by environmental DNA metabarcoding6) across 46 proglacial landscapes worldwide, we found that all the environmental properties change with time since glaciers retreated, and that temperature modulates the accumulation of soil nutrients. The richness of bacteria, fungi, plants and animals increases with time since deglaciation, but their temporal patterns differ. Microorganisms colonized most rapidly in the first decades after glacier retreat, whereas most macroorganisms took longer. Increased habitat suitability, growing complexity of biotic interactions and temporal colonization all contribute to the increase in biodiversity over time. These processes also modify community composition for all the groups of organisms. Plant communities show positive links with all other biodiversity components and have a key role in ecosystem development. These unifying patterns provide new insights into the early dynamics of deglaciated terrains and highlight the need for integrated surveillance of their multiple environmental properties5.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw sequence data and filtered sequence data are available at https://doi.org/10.5281/zenodo.6620359 and https://doi.org/10.5281/zenodo.10423968 digital repositories, respectively.
Code availability
All codes used are available at https://doi.org/10.5281/zenodo.10423968.
References
Ficetola, G. F. et al. Dynamics of ecological communities following current retreat of glaciers. Annu. Rev. Ecol. Evol. Syst. 52, 405–426 (2021).
Pothula, S. K. & Adams, B. J. Community assembly in the wake of glacial retreat: a meta-analysis. Glob. Chang. Biol. 28, 6973–6991 (2022).
Bosson, J. B. et al. Future emergence of new ecosystems caused by glacial retreat. Nature 620, 562–569 (2023).
Rounce, D. R. et al. Global glacier change in the 21st century: every increase in temperature matters. Science 379, 78–83 (2023).
Zimmer, A., Beach, T., Klein, J. A. & Recharte Bullard, J. The need for stewardship of lands exposed by deglaciation from climate change. Wiley Interdiscip. Rev. Clim. Change 13, e753 (2022).
Taberlet, P., Bonin, A., Zinger, L. & Coissac, E. Environmental DNA: For Biodiversity Research and Monitoring (Oxford Univ. Press, 2018).
Hock, R. et al. GlacierMIP — a model intercomparison of global-scale glacier mass-balance models and projections. J. Glaciol. 65, 453–467 (2019).
Lee, J. R. et al. Climate change drives expansion of Antarctic ice-free habitat. Nature 547, 49–54 (2017).
Körner, C. Mountain biodiversity, its causes and function. Ambio 33, 11–17 (2004).
Palomo, I. Climate change impacts on ecosystem services in high mountain areas: a literature review. Mt. Res. Dev. 37, 179–187 (2017).
La Farge, C., Williams, K. H. & England, J. H. Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments. Proc. Natl Acad. Sci. USA 110, 9839–9844 (2013).
Donhauser, J. & Frey, B. Alpine soil microbial ecology in a changing world. FEMS Microbiol. Ecol. 94, fiy099 (2018).
Hågvar, S. et al. Ecosystem birth near melting glaciers: a review on the pioneer role of ground-dwelling arthropods. Insects 11, 644 (2020).
Cauvy-Fraunié, S. & Dangles, O. A global synthesis of biodiversity responses to glacier retreat. Nat. Ecol. Evol. 3, 1675–1685 (2019).
Hugonnet, R. et al. Accelerated global glacier mass loss in the early twenty-first century. Nature 592, 726–731 (2021).
Moore, J. W. et al. Mining stakes claim on salmon futures as glaciers retreat. Science 382, 887–889 (2023).
Poorter, L. et al. Multidimensional tropical forest recovery. Science 374, 1370–1376 (2021).
Walker, L. R., Wardle, D. A., Bardgett, R. D. & Clarkson, B. D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736 (2010).
Connell, J. H. & Slatyer, R. O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 111, 1119–1144 (1977).
Hanusch, M., He, X., Ruiz-Hernández, V. & Junker, R. R. Succession comprises a sequence of threshold-induced community assembly processes towards multidiversity. Commun. Biol. 5, 424 (2022).
Pulsford, S. A., Lindenmayer, D. B. & Driscoll, D. A. A succession of theories: purging redundancy from disturbance theory. Biol. Rev. 91, 148–167 (2016).
Rosero, P. et al. Multi-taxa colonisation along the foreland of a vanishing equatorial glacier. Ecography 44, 1010–1021 (2021).
Fan, K. et al. Soil biodiversity supports the delivery of multiple ecosystem functions in urban greenspaces. Nat. Ecol. Evol. 7, 113–126 (2023).
Khedim, N. et al. Topsoil organic matter build-up in glacier forelands around the world. Glob. Chang. Biol. 27, 1662–1677 (2021).
Lutz, S. et al. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nat. Commun. 7, 11968 (2016).
Rime, T., Hartmann, M. & Frey, B. Potential sources of microbial colonizers in an initial soil ecosystem after retreat of an alpine glacier. ISME J. 10, 1625–1641 (2016).
Zimmer, A. et al. Soil temperature and local initial conditions drive carbon and nitrogen build-up in young proglacial soils in the Tropical Andes and European Alps. Catena 235, 107645 (2024).
Bardgett, R. D. et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 3, 487–490 (2007).
Hunter, B. D., Roering, J. J., Silva, L. C. R. & Moreland, K. C. Geomorphic controls on the abundance and persistence of soil organic carbon pools in erosional landscapes. Nat. Geosci. 17, 151–157 (2024).
Draebing, D., Mayer, T., Jacobs, B. & McColl, S. T. Alpine rockwall erosion patterns follow elevation-dependent climate trajectories. Commun. Earth Environ. 3, 21 (2022).
Erhart, H. La Génèse Des Sols En Tant Que Phénomène Géologique: Esquisse d’une Théorie Géologique et Géochimique: Biostasie et Rhexistasie (Masson, 1951).
Salazar, A., Warshan, D., Vasquez-Mejia, C. & Andrésson, Ó. S. Environmental change alters nitrogen fixation rates and microbial parameters in a subarctic biological soil crust. Oikos 2022, e09239 (2022).
Sepp, S.-K. et al. Global diversity and distribution of nitrogen-fixing bacteria in the soil. Front. Plant Sci. 14, 1100235 (2023).
Bahram, M. et al. Structure and function of the global topsoil microbiome. Nature 560, 233–237 (2018).
Angert, A. L., Huxman, T. E., Chesson, P. & Venable, D. L. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl Acad. Sci. USA 106, 11641–11645 (2009).
Peyre, G. et al. The fate of páramo plant assemblages in the sky islands of the northern Andes. J. Veg. Sci. 31, 967–980 (2020).
Vellend, M. et al. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123, 1420–1430 (2014).
Martiny, J. B. H. et al. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112 (2006).
Ohlmann, M. et al. Mapping the imprint of biotic interactions on β-diversity. Ecol. Lett. 21, 1660–1669 (2018).
Tscherko, D., Hammesfahr, U., Zeltner, G., Kandeler, E. & Böcker, R. Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain. Basic Appl. Ecol. 6, 367–383 (2005).
Losapio, G. et al. Network motifs involving both competition and facilitation predict biodiversity in alpine plant communities. Proc. Natl Acad. Sci. USA 118, e2005759118 (2021).
Sint, D., Kaufmann, R., Mayer, R. & Traugott, M. Resolving the predator first paradox: arthropod predator food webs in pioneer sites of glacier forelands. Mol. Ecol. 28, 336–347 (2019).
Bennett, J. A. et al. Plant–soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184 (2017).
Calderón-Sanou, I. et al. Cascading effects of moth outbreaks on subarctic soil food webs. Sci. Rep. 11, 15054 (2021).
Houlton, B. Z., Wang, Y.-P., Vitousek, P. M. & Field, C. B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454, 327–330 (2008).
Tedersoo, L., Bahram, M. & Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 367, eaba1223 (2020).
Cantera, I. et al. The importance of species addition ‘versus’ replacement varies over succession in plant communities after glacier retreat. Nat. Plants 10, 256–267 (2024).
Pugnaire, F. I. et al. Climate change effects on plant–soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 5, eaaz1834 (2019).
Guerra, C. A. et al. Global hotspots for soil nature conservation. Nature 610, 693–698 (2022).
Sytsma, M. L. T., Lewis, T., Bakker, J. D. & Prugh, L. R. Successional patterns of terrestrial wildlife following deglaciation. J. Anim. Ecol. 92, 723–737 (2023).
Butler, D. R., Anzah, F., Goff, P. D. & Villa, J. Zoogeomorphology and resilience theory. Geomorphology 305, 154–162 (2018).
Zemp, M. et al. Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature 568, 382–386 (2019).
Marta, S. et al. The retreat of mountain glaciers since the Little Ice Age: a spatially explicit database. Data 6, 107 (2021).
Dickie, I. A. et al. Towards robust and repeatable sampling methods in eDNA-based studies. Mol. Ecol. Resour. 18, 940–952 (2018).
Guerrieri, A. et al. Metabarcoding data reveal vertical multitaxa variation in topsoil communities during the colonization of deglaciated forelands. Mol. Ecol. https://doi.org/10.1111/mec.16669 (2023).
Rime, T. et al. Vertical distribution of the soil microbiota along a successional gradient in a glacier forefield. Mol. Ecol. 24, 1091–1108 (2015).
Guerrieri, A. et al. Effects of soil preservation for biodiversity monitoring using environmental DNA. Mol. Ecol. 30, 3313–3325 (2021).
Bray, R. H. & Kurtz, L. T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 59, 39–46 (1945).
Olsen, S. R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (US Department of Agriculture, 1954).
Marta, S. et al. Heterogeneous changes of soil microclimate in high mountains and glacier forelands. Nat. Commun. https://doi.org/10.21203/rs.3.rs-2017904/v1 (2023).
Smith, P. & Metcalfe, P. dynatop: An implementation of dynamic TOPMODEL hydrological model in R. GitHub https://github.com/waternumbers/dynatop (2022).
Paruelo, J. M., Epstein, H. E., Lauenroth, W. K. & Burke, I. C. ANPP estimates from NDVI for the central grassland region of the United States. Ecology 78, 953–958 (1997).
Rumpf, S. B. et al. From white to green: snow cover loss and increased vegetation productivity in the European Alps. Science 376, 1119–1122 (2022).
Lillesand, T., Kiefer, R. W. & Chipman, J. Remote Sensing and Image Interpretation 7th edn (Wiley, 2015).
Liu, Y. et al. Evaluation of consistency among three NDVI products applied to High Mountain Asia in 2000–2015. Remote Sens. Environ. 269, 112821 (2022).
Aybar, C. et al. rgee: R bindings for calling the ‘Earth Engine’ API. GitHub https://github.com/google/earthengine-api (2022).
Ficetola, G. F. & Taberlet, P. Towards exhaustive community ecology via DNA metabarcoding. Mol. Ecol. https://doi.org/10.1111/mec.16881.
Guardiola, M. et al. Deep-sea, deep-sequencing: metabarcoding extracellular DNA from sediments of marine canyons. PLoS ONE 10, e0139633 (2015).
Moll, J. & Hoppe, B. Evaluation of primers for the detection of deadwood-inhabiting archaea via amplicon sequencing. PeerJ 10, e14567 (2022).
Hathaway, J. J. M., Moser, D. P., Blank, J. G. & Northup, D. E. A comparison of primers in 16S rRNA gene surveys of Bacteria and Archaea from volcanic caves. Geomicrobiol. J. 38, 741–754 (2021).
Epp, L. S. et al. New environmental metabarcodes for analysing soil DNA: potential for studying past and present ecosystems. Mol. Ecol. 21, 1821–1833 (2012).
Taberlet, P. et al. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 35, e14 (2007).
Janssen, P. et al. Present conditions may mediate the legacy effect of past land-use changes on species richness and composition of above- and below-ground assemblages. J. Ecol. 106, 306–318 (2018).
Bienert, F. et al. Tracking earthworm communities from soil DNA. Mol. Ecol. 21, 2017–2030 (2012).
Lunghi, E. et al. Environmental DNA of insects and springtails from caves reveals complex processes of eDNA transfer in soils. Sci. Total Environ. 826, 154022 (2022).
Coissac, E. OligoTag: a program for designing sets of tags for next-generation sequencing of multiplexed samples. Methods Mol. Biol. 888, 13–31 (2012).
Zinger, L. et al. DNA metabarcoding — need for robust experimental designs to draw sound ecological conclusions. Mol. Ecol. 28, 1857–1862 (2019).
Boyer, F. et al. obitools: A unix-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 16, 176–182 (2016).
Brown, S. P. et al. Scraping the bottom of the barrel: are rare high throughput sequences artifacts? Fungal Ecol. 13, 221–225 (2015).
Alberdi, A., Aizpurua, O., Gilbert, M. T. P. & Bohmann, K. Scrutinizing key steps for reliable metabarcoding of environmental samples. Methods Ecol. Evol. 9, 134–147 (2018).
Bonin, A., Guerrieri, A. & Ficetola, G. F. Optimal sequence similarity thresholds for clustering of molecular operational taxonomic units in DNA metabarcoding studies. Mol. Ecol. Resour. 23, 368–381 (2023).
Calderón‐Sanou, I., Münkemüller, T., Boyer, F., Zinger, L. & Thuiller, W. From environmental DNA sequences to ecological conclusions: how strong is the influence of methodological choices? J. Biogeogr. 47, 193–206 (2020).
Bálint, M. et al. Millions of reads, thousands of taxa: microbial community structure and associations analyzed via marker genes. FEMS Microbiol. Rev. 40, 686–700 (2016).
Ficetola, G. F. et al. Replication levels, false presences and the estimation of the presence/absence from eDNA metabarcoding data. Mol. Ecol. Resour. 15, 543–556 (2015).
Ariza, M. et al. Plant biodiversity assessment through soil eDNA reflects temporal and local diversity. Methods Ecol. Evol. 14, 415–430 (2023).
Pansu, J. et al. Long-lasting modification of soil fungal diversity associated with the introduction of rabbits to a remote sub-Antarctic archipelago. Biol. Lett. 11, 20150408 (2015).
Foucher, A. et al. Persistence of environmental DNA in cultivated soils: implication of this memory effect for reconstructing the dynamics of land use and cover changes. Sci. Rep. 10, 10502 (2020).
O’Malley, M. A., Simpson, A. G. B. & Roger, A. J. The other eukaryotes in light of evolutionary protistology. Biol. Philos. 28, 299–330 (2013).
Whittaker, R. H. New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science 163, 150–160 (1969).
Simpson, A. G. B., Slamovits, C. H. & Archibald, J. M. in Handbook of the Protists (eds Archibald, J. M., Simpson, A. G. B. & Slamovits, C. H.) 1–21 (Springer International, 2017).
Anthony, M. A., Bender, S. F. & van der Heijden, M. G. A. Enumerating soil biodiversity. Proc. Natl Acad. Sci. USA 120, e2304663120 (2023).
Fierer, N., Strickland, M. S., Liptzin, D., Bradford, M. A. & Cleveland, C. C. Global patterns in belowground communities. Ecol. Lett. 12, 1238–1249 (2009).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Zinger, L. et al. Body size determines soil community assembly in a tropical forest. Mol. Ecol. 28, 528–543 (2019).
Johnson, E. A. & Miyanishi, K. Testing the assumptions of chronosequences in succession. Ecol. Lett. 11, 419–431 (2008).
Makoto, K. & Wilson, S. D. New multicentury evidence for dispersal limitation during primary succession. Am. Nat. 187, 804–811 (2016).
Rydgren, K., Halvorsen, R., Töpper, J. P. & Njøs, J. M. Glacier foreland succession and the fading effect of terrain age. J. Veg. Sci. 25, 1367–1380 (2014).
Tampucci, D. et al. Plant and arthropod colonisation of a glacier foreland in a peripheral mountain range. Biodiversity 16, 213–223 (2015).
Vater, A. E. & Matthews, J. A. Succession of pitfall-trapped insects and arachnids on eight Norwegian glacier forelands along an altitudinal gradient: patterns and models. Holocene 25, 108–129 (2015).
Damgaard, C. A critique of the space-for-time substitution practice in community ecology. Trends Ecol. Evol. 34, 416–421 (2019).
Smith, J. et al. BioDeepTime: a database of biodiversity time series for modern and fossil assemblages. Global Ecol. Biogeogr. 32, 1680–1689 (2023).
Foster, B. L. & Tilman, D. Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol. 146, 1–10 (2000).
Erschbamer, B., Niederfriniger Schlag, R., Carnicero, P. & Kaufmann, R. Long-term monitoring confirms limitations of recruitment and facilitation and reveals unexpected changes of the successional pathways in a glacier foreland of the Central Austrian Alps. Plant Ecol. 224, 373–386 (2023).
Fickert, T. & Grüninger, F. High-speed colonization of bare ground — permanent plot studies on primary succession of plants in recently deglaciated glacier forelands. Land Degrad. Dev. 29, 2668–2680 (2018).
Mächler, E., Walser, J.-C. & Altermatt, F. Decision-making and best practices for taxonomy-free environmental DNA metabarcoding in biomonitoring using Hill numbers. Mol. Ecol. 30, 3326–3339 (2021).
McMurdie, P. J. & Holmes, S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 10, e1003531 (2014).
Bürkner, P.-C. brms: An R package for bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
Ren, Z. & Gao, H. Abundant and rare soil fungi exhibit distinct succession patterns in the forefield of Dongkemadi glacier on the central Qinghai-Tibet Plateau. Sci. Total Environ. 828, 154563 (2022).
Bjornstad, O. N. & Cai, J. ncf: Spatial covariance functions. CRAN https://doi.org/10.32614/CRAN.package.ncf (2022).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems (Springer Nature, 2021).
Paulsen, J. & Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Botany 124, 1–12 (2014).
Lefcheck, J. S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016).
Delavaux, C. S., Ramos, R. J., Sturmer, S. L. & Bever, J. D. Environmental identification of arbuscular mycorrhizal fungi using the LSU rDNA gene region: an expanded database and improved pipeline. Mycorrhiza 32, 145–153 (2022).
Delavaux, C. S. et al. Mycorrhizal types influence island biogeography of plants. Commun. Biol. 4, 1128 (2021).
Bollen, K. A., Harden, J. J., Ray, S. & Zavisca, J. BIC and alternative Bayesian information criteria in the selection of structural equation models. Struct. Equ. Modeling 21, 1–19 (2014).
Hertzog, L. R. How robust are structural equation models to model miss-specification? A simulation study. Preprint at arXiv https://doi.org/10.48550/arXiv.1803.06186 (2019).
Lin, L.-C., Huang, P.-H. & Weng, L.-J. Selecting path models in SEM: a comparison of model selection criteria. Struct. Equ. Modeling 24, 855–869 (2017).
Oberski, D. lavaan.survey: An R package for complex survey analysis of structural equation models. J. Stat. Softw. 57, 1–27 (2014).
Shipley, B. & Douma, J. C. Generalized AIC and chi-squared statistics for path models consistent with directed acyclic graphs. Ecology 101, e02960 (2020).
Douma, J. C. & Shipley, B. Testing model fit in path models with dependent errors given non-normality, non-linearity and hierarchical data. Struct. Equ. Modeling 30, 222–233 (2023).
Westland, J. C. Structural Equation Models: From Paths to Networks (Springer, 2020).
Dormann, C. F. et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 (2007).
Lichstein, J., Simons, T., Shriner, S. & Franzreb, K. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 72, 445–463 (2002).
Roser, L. G., Ferreyra, L. I., Saidman, B. O. & Vilardi, J. C. EcoGenetics: an R package for the management and exploratory analysis of spatial data in landscape genetics. Mol. Ecol. Resour. 17, e241–e250 (2017).
Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference with R (Cambridge Univ. Press, 2016).
Legendre, P., Lapointe, F.-J. & Casgrain, P. Modeling brain evolution from behavior: a permutational regression approach. Evolution 48, 1487–1499 (1994).
Martinez-Almoyna, C. et al. Multi-trophic β-diversity mediates the effect of environmental gradients on the turnover of multiple ecosystem functions. Funct. Ecol. 33, 2053–2064 (2019).
Lichstein, J. W. Multiple regression on distance matrices: a multivariate spatial analysis tool. Plant Ecol. 188, 117–131 (2007).
Acknowledgements
This study was funded by the European Research Council under the European Community’s Horizon 2020 Programme, grant agreement no. 772284 (IceCommunities), by Biodiversa+, the European Biodiversity Partnership under the 2021–2022 BiodivProtect joint call for research proposals, co-funded by the European Commission (no. 101052342) and with the funding organizations MUR and ANR, and by LabEx OSUG@2020 (Investissement d’Avenir, ANR-10-LABX-56). P.C. was supported by the Science and Engineering Research Board (SERB), Department of Science and Technology (GoI), NPDF project no. PDF/2017/002717. L.T. was also supported by the Australian Research Council Special Research Initiative Securing Antarctica’s Environmental Future (no. SR200100005). Y.Y. was supported by the National Natural Science Foundation of China (41941015). D.F. was supported by the National Biodiversity Future Center of the National Recovery and Resilience Plan (no. CN_00000033).
Author information
Authors and Affiliations
Contributions
G.F.F. conceived the work with the help of W.T., P.T. and J.P. G.F.F., S.M., A.G., A.B., R.A., M.C., F.A., R.S.A., P.A., P.A.G., S.C.-F., J.L.C.L, P.C., M.C.S., J.J.C., J.A.C.R., C.C., R.C.E., O.D., P.D., A.E., S.E., A.F., L.G., F.G., M.G., S.H., R.K., N.K., R.I.M., M.A.M.-M., G.P., F.P., A.R., K.S., L.T., N.U., Y.Y., V.Z., A.Zimmer, G.A.D. and J.P. planned the data collection and performed the sampling. A.G., A.B., C.C., L.G., A.P., A.Zerboni and G.F.F. performed the laboratory analyses. A.C., S.M., A.G., I.C., D.F., W.T. and G.F.F. contributed to data preparation and statistical analyses. A.C. and G.F.F. prepared the first draft of the manuscript, with subsequent contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Peter Convey, Jacob C. Douma, Arwyn Edwards, Nicolas Lecomte, Lawrence Tanner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
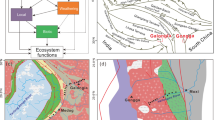
Extended Data Fig. 1 Study sites and sampling design.
a, Global distribution of the 46 analysed proglacial landscapes. The size of symbols is proportional to the average number of detected MOTUs per plot. The inset shows the landscapes in the European Alps. b, Sampling design. For each proglacial landscape, we selected a number of sites corresponding to the lines representing the position of the glacier forefront at a given date (following ref. 53; four sites are represented in this example). For each site, we established five regularly spaced plots (diamonds; distance between plots: 20 m); at each plot, we collected five soil subsamples within a 1-m radius and pooled subsamples together, resulting in a ~ 200 g composite sample per plot (total: 46 landscapes; 256 sites; 1,256 plots analysed separately).
Extended Data Fig. 2 Pearson’s correlations between the alpha-diversity of eight taxonomic groups of organisms within proglacial landscapes.
Larger dots and more intense colours indicate stronger correlations. N = 1,251 plots.
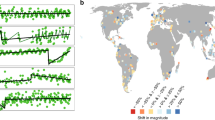
Extended Data Fig. 3 Relationship between diversity values predicted by chronosequence-based models, and diversity values observed in permanent plots surveyed in multiple years by independent studies.
These studies are refs. 103,104. The bold line indicates values predicted by a mixed model relating observed and predicted diversity (marginal R2 = 0.43, conditional R2 = 0.63); the shaded area represents the 95% credible intervals. Diversity was measured using Hill’s number q = 1. Differences in absolute values are related to methodological differences between our sampling approach and independent data used for validation. For instance, ref. 103 provided total diversity across 10 plots spread over 150 m2 on each site; ref. 104 calculated diversity across 11-13 plots per site, while our diversity predictions refer to one plot per site.
Extended Data Fig. 4 Differences in colonization rate of eight groups of organisms between subpolar, temperate and tropical landscapes.
a–h, The plots represent the relationship between age and diversity of the groups estimated by mixed models (Supplementary Table 2); error bars represent 95% credible intervals. i, Average soil temperature during the growing season in the 1,251 analysed plots.
Extended Data Fig. 5 Conceptual models representing how time and microclimate can drive soil chemistry and (alpha or beta) biodiversity changes in ecological succession following glacier retreat.
To determine the relationships between soil nutrients and biodiversity, we tested three conceptual models assessing three potential causal structures, either (a) soil nutrients and ecosystem productivity shape biodiversity, (b) biodiversity shapes soil nutrients and productivity, or (c) soil nutrients, productivity and biodiversity co-vary. “Soil biodiversity” indicates the biodiversity of all the organisms beside plants (i.e. bacteria, fungi, protists and animals). The detailed structure of models, including the relationships between nutrients, plants and productivity, is shown in Fig. 2.
Extended Data Fig. 6 Alternative structural equation models, assuming different relationships between alpha-diversity, soil nutrients and ecosystem productivity.
The colour of the paths in a–c is proportional to effect size; dashed lines indicate non-significant relationships. Co-variations between soil features and between the biodiversity of different taxonomic groups are not shown. In all the models, N = 793 plots.
Extended Data Fig. 7 Relationship between the number of molecular operational taxonomic units (MOTUs) detected by environmental DNA (eDNA) and the number of species recorded in traditional inventories for plants and insects.
For plants, N = 38 sites from 10 forelands; for insects, N = 44 sites from 13 forelands. We show the partial regression plot of linear mixed models accounting for glacier identity (plants: R2C = 0.86; insects: R2C = 0.83). For eDNA we used the number of MOTUs detected on soil samples across all plots in a site; for traditional detections we used the number of taxa identified at species and genus level. See Supplementary Table 6 for the sources of traditional data.
Supplementary information
Supplementary Information
This Supplementary Information file includes: Supplementary Tables 1–12, Supplementary Figures 1–3, and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ficetola, G.F., Marta, S., Guerrieri, A. et al. The development of terrestrial ecosystems emerging after glacier retreat. Nature 632, 336–342 (2024). https://doi.org/10.1038/s41586-024-07778-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07778-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.