Abstract
The region with the highest marine biodiversity on our planet is known as the Coral Triangle or Indo-Australian Archipelago (IAA)1,2. Its enormous biodiversity has long attracted the interest of biologists; however, the detailed evolutionary history of the IAA biodiversity hotspot remains poorly understood3. Here we present a high-resolution reconstruction of the Cenozoic diversity history of the IAA by inferring speciation–extinction dynamics using a comprehensive fossil dataset. We found that the IAA has exhibited a unidirectional diversification trend since about 25 million years ago, following a roughly logistic increase until a diversity plateau beginning about 2.6 million years ago. The growth of diversity was primarily controlled by diversity dependency and habitat size, and also facilitated by the alleviation of thermal stress after 13.9 million years ago. Distinct net diversification peaks were recorded at about 25, 20, 16, 12 and 5 million years ago, which were probably related to major tectonic events in addition to climate transitions. Key biogeographic processes had far-reaching effects on the IAA diversity as shown by the long-term waning of the Tethyan descendants versus the waxing of cosmopolitan and IAA taxa. Finally, it seems that the absence of major extinctions and the Cenozoic cooling have been essential in making the IAA the richest marine biodiversity hotspot on Earth.
Similar content being viewed by others
Main
It is unclear how the global centre of marine biodiversity, the IAA, has developed, and why its biodiversity is disproportionally high compared to that of other tropical regions. These are key uncertainties in organismal biology. We have gradually gained a better understanding of global-scale diversity dynamics throughout the Cenozoic4,5, revealing complex waxing and waning of diversity related to climatic and other environmental changes. However, regionally resolved Cenozoic diversity trends remain poorly understood owing to the scarcity of historical data and their compilation. This is particularly true for the tropics in deeper time, making the origins of high biodiversity an enigma6,7,8. The current knowledge of the fossil record suggests that the locations of peak diversity (that is, biodiversity hotspots) shifted throughout the Cenozoic from the western Tethys during the Eocene to the Arabian Peninsula during the late Eocene–Oligocene, before being established at the current location of the IAA in Southeast Asia in the early Miocene: the process known as the hopping hotspots model3,9. Plate tectonics is postulated to be the ultimate driver of this process by regulating the broad-scale availability and configuration of shallow-marine habitats with successive continent collisions9. Each hop of the biodiversity hotspots from the ancient location to the new one was probably underpinned by considerable speciation and extinction events, but also could be associated with the palaeobiogeographic shifts of some component taxa tracking suitable habitats3. However, the detailed Cenozoic history of the IAA hotspot remains elusive as explicated below. Better understanding the deep-time origin, evolution and maintenance of this most diverse place in the marine realm is crucial for macroevolutionary and macroecological studies and provides a solid theoretical framework for conservation efforts.
As one of the most conspicuous biogeographic and biodiversity patterns today, the IAA hotspot is characterized by an exceptional concentration of coastal benthic species, whereas pelagic groups show widespread distributions without a distinguished centre of diversity2,10. Historical evidence from benthic taxonomic groups has advanced our understanding of tropical diversification yet suffers from various limitations. Recent molecular studies on corals and reef fishes revealed their biogeographic and evolutionary history to build the IAA hotspot, suggesting that it was a centre of species accumulation and origin at different intervals of the Cenozoic11,12. However, these phylogeny-based models are subject to uncertainties in the case of incomplete sampling and undocumented extinctions. They also are not geographically defined to quantify a clear scenario of regional diversity changes in the IAA. In addition to molecular phylogeny evidence, previous palaeontological studies collectively indicate a compatible trend of increased IAA species richness at a coarse spatiotemporal resolution, which was dominated by immigration into the IAA in the Eocene–Oligocene and proliferation inside the IAA in the Oligocene–recent6,9,13,14,15. However, difficulties in reconstructing a more detailed IAA history for benthic fossil groups include: a lack of sufficient fossil data or data synthesis for molluscs and bryozoans8,15; a small species pool despite a good fossil record for larger benthic foraminifera6; and uncertainties in species-level identification for fossil corals7. Another critical, yet often overlooked, limitation is that our current knowledge of the marine hotspot is severely biased towards (sub)tropical reef-associated groups (mostly corals and reef fishes), even though the IAA peak in species richness is a common feature shared by many shallow-marine lineages regardless of reef affiliation10. By relying on reef taxa as the sole descriptor of the IAA hotspot, the strong correlation of their diversity patterns with reef habitat may mask the effects of other putative primary drivers on macroevolutionary and macroecological dynamics. Overall, Ostracoda (Arthropoda: Crustacea; known as seed shrimps) is one of the few benthic microfossil organisms that has left a rich fossil record within and beyond reef ecosystems for quantitative analysis16. Their high species diversity and robust taxonomy are two other major advantages16. Benthic ostracods show a normal latitudinal diversity gradient17 and depth diversity gradient18. They also exhibit a similar biogeographic distribution with other invertebrates19. Thus, instead of being a contrarian, ostracods are regarded as a normal benthic taxon that tends to follow standard ecological patterns17. In addition, small (<0.5 mm) benthic metazoan invertebrates account for most (more than two-thirds of) marine biodiversity17,20, and the ostracod is probably the best fossil representative for this group in terms of general biotic response13,16. These features make ostracods a very useful proxy for broad marine benthic biodiversity to investigate the ancient history of hotspots before the timescale of modern observations13,16.
Here we used ostracods as a model proxy to assemble the first comprehensive Cenozoic fossil dataset for the IAA hotspot. Applying a birth–death model that incorporates the preservation process and mitigates bias in the fossil record, we first inferred a detailed regional diversity trajectory by explicitly estimating speciation and extinction rates. Given the regional scope of this study, speciation here corresponds to the first appearance of every species in the IAA to construct the emerging hotspot. The same rationale applies to extinction, which is defined as the final extirpation of any species from the IAA instead of global extinction. We then correlated the IAA’s macroevolutionary dynamics with a set of biotic and abiotic parameters to assess the potential biodiversity drivers. Finally, we placed the evolution of the IAA hotspot in a biogeographic context to explore how key biogeographic processes (that is, migration and origination of the Tethyan, cosmopolitan and endemic IAA fauna) altered long-term diversification.
Cenozoic diversity history of the IAA
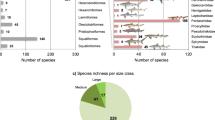
We first assembled a Cenozoic fossil ostracod dataset from 216 samples across the IAA region (Extended Data Fig. 1), totalling 47,727 specimens for 874 morphospecies (including 94 spp. entries in which at least a part of a genus was impossible to identify at species level and was treated as 1 species entry apiece in the analyses). We estimated the best-fit model that accounts for the preservation rates of the dataset, which was a non-homogeneous Poisson process (significance at P < 0.01), in which preservation rates change over the lifetime of each lineage according to a bell-shaped distribution (median estimate of 45.04 occurrences per million years per taxon). Through a Bayesian process-based birth–death analysis of our integrated dataset, we quantitatively documented a detailed Cenozoic diversity history of the IAA hotspot, revealing the generation and maintenance of its exceptional high diversity (Fig. 1). Species richness was very low during most of the Palaeogene, consistent with the hypothesis that the IAA was not the place of a biodiversity hotspot during this period. With an initial increase in diversity in the late Oligocene, in line with the hopping hotspots model9, rapid diversification began about 25 million years ago (Ma), making the IAA a rising hotspot. These early diversifications are probably broadly related to the collision of the southeast Eurasian margin with the Australian and Pacific plates and the resulting development of the vast area of complex habitat in the IAA3,9,21. Thereafter, strong diversification continued throughout the Miocene–Pliocene and culminated in the Pleistocene about 2.6 Ma, when species richness increased more than sixfold relative to the Eocene average, reaching an exceptionally high value of more than 650 species, and finally exhibiting a diversity plateau until the present. In addition to the long-term trends, speciation rates peaked at about 25, 20, 16, 12 and 5 Ma, corresponding to phases of rapid increase in the diversity curve. Extinction rates remained comparatively low throughout the Cenozoic, except for four peaks consistent with those of speciation rates at about 25, 20, 16 and 5 Ma. Nevertheless, it should be noted that there is a major sampling gap across the early Oligocene, so the possibility of an earlier diversification before 25 Ma cannot be completely ruled out7,14. Our raw analyses of the ostracod dataset, based on rarefaction and species range plots, indicated a consistent pattern of striking diversity increase at each geological interval from the early Miocene until the Pleistocene (Extended Data Figs. 2 and 3 and Supplementary Table 1). Within the IAA, the Philippines emerged as the bull’s-eye of ostracod diversity from the late Miocene to Pleistocene, which is congruent with modern distributions of overall marine species richness10,22 (Extended Data Fig. 4).
a–d, Bayesian inferences of speciation rates per lineage (a), extinction rates per lineage (b), net diversification rates as the difference between speciation and extinction rates (rates below 0 indicate declining diversity; c) and species richness of all ostracods (d). Solid lines indicate mean posterior rates, and the shaded areas show the 95% confidence interval. E, Eocene; O, Oligocene; EM, early Miocene; MM, middle Miocene, LM, late Miocene; P1, Pliocene; P2, Pleistocene. Early Oligocene, early Miocene, late Miocene and Pleistocene are shaded.
Most noticeably, extinction occurred at very low background rates in the IAA throughout its Cenozoic history, except for the small and discrete peaks as mentioned in the last paragraph (Fig. 1b). Our results are in agreement with a previous study that showed that huge spatial differences in extinction rates between the IAA and Caribbean may be crucial for the modern longitudinal diversity patterns across the tropical belt, with the largest hotspot situated in the IAA in contrast to much lower diversity in the Caribbean8. As two major hotspots of the early Neogene in similar tectonic and oceanographic settings, the IAA and the Caribbean may exhibit parallel processes of diversification during the Miocene to encompass comparable levels of species richness3,8. The modern disparity in diversity between the two regions may have developed during the Plio-Pleistocene with the final closure of the Central American Seaway between 4 and 2 Ma triggering the Caribbean mass extinction3,23. Such a catastrophic event terminated the Caribbean hotspot, whereas our analysis suggests that a longer trend of diversification continued smoothly in the IAA after the Miocene to promote modern-scale species richness. Thus, the absence of mass extinction is a prerequisite for the development and maturation of the IAA hotspot as a global centre of diversity under long-term stable and favourable palaeoenvironmental conditions across the Neogene and Quaternary. The present-day global tropical diversity pattern may be primarily shaped by deep-time extinctions, suggesting that the study of modern diversity and environments alone is not sufficient to fully understand the biosphere of our planet.
Drivers of the Cenozoic diversification
We deciphered potential drivers of IAA’s long-term diversity dynamics by simultaneously assessing the effects of key biotic (diversity dependency) and abiotic (habitat size and complexity, temperature and sea level) factors, using a multivariate birth–death (MBD) model spanning the entire time frames (that is, Eocene–present). The abiotic variables chosen here reflect the changes in tectonics, climate and oceanography that may have shaped the most fundamental aspects of Earth’s physical environment and played an overarching role in determining broad biological outcomes in the tropics1,3. First, we revealed a substantial degree of diversity dependence within ostracod assemblages, with a strong negative correlation between diversity and speciation rate (correlation parameter G = −2.735, strength of correlation parameter ω = 0.863; Fig. 2a and Supplementary Table 2). Accordingly, we observe a long-term decrease in speciation rate and an approximately logistic growth of diversity for the Neogene–Quaternary development of the IAA hotspot (Fig. 1a,d), which is consistent with the bounded diversity hypothesis24,25,26. Indeed, lineages and communities may commonly experience a slowdown in their diversification rates coupled with a damped increase in diversity. Higher numbers of species may indicate increased biotic (competitive) interactions and expanded niche occupancy in a region, which conversely reduces ecological opportunities for speciation24,26. Finite resources, ultimately determined by extrinsic factors (for example, climate and regional area), may result in an upper limit to the niche space shared by all species, and thus hypothetically limit the maximum diversity that can be achieved as ‘carrying capacity’24. Bounded diversification seems to be a recurrent and pervasive pattern in macroevolutionary studies across different times, places and taxa, although empirical evidence for diversity truly exhausting its potential to increase with niche saturation remains scarce25. In our case of the IAA hotspot, it seems that long-term diversification since about 25 Ma has led to a steady, asymptotic phase of equilibrium diversity following the Plio-Pleistocene transition about 2.6 Ma. This may represent the maturation of the IAA hotspot after a long Neogene history of expansion, but the possibility of future diversity growth remains if the carrying capacity increases in response to changing environmental conditions, or if a key innovation evolves to conquer new niche space25,26, which would make the current phase only a diversity plateau in the hotspot’s life cycle.
a–d, Bayesian inferences of correlation parameters on speciation (red) and extinction (blue) with abiotic factors including global temperature, global sea level, IAA habitat size (shelf area) and IAA habitat complexity (coastline length), and diversity dependence factor (that is, the diversity over time of the entire ostracod assemblage), for the time frame of the entire Cenozoic (a), Neogene warm phase (23.04–13.9 Ma; b), Neogene cooling phase (13.9–5.33 Ma; c) and Neogene–Quaternary cold phase (5.33–0 Ma; d). The asterisk indicates a significant correlation parameter for a given variable (shrinkage weights ω > 0.5).
In addition to diversity dependence that imposed a strong biotic control on the IAA, we showed that habitat size (shelf area) as the most important abiotic determinant has a positive, albeit weak, effect on speciation, indicating an evolutionary species–area relationship (G = 2.272 × 10−7, ω = 0.647; Fig. 2a and Supplementary Table 2). Larger shelf areas could promote speciation either through direct effects of area or through the effects of factors that are highly correlated with area, such as population size, species range and environmental heterogeneity27,28. The Neogene expansion of shallow-marine habitats in the IAA, which was driven by the prolonged collisions between Southeast Asia and Australia21,29, was therefore pivotal in the rise of the IAA hotspot. Unexpectedly, habitat complexity (coastline length) as another putative diversification driver has no effect here (insignificant correlation with both speciation and extinction rates; ω = 0.272; Fig. 2a and Supplementary Table 2). Our results seem to contradict previous theories suggesting that complex island archipelagos of the IAA with an extensive array of shallow seas accelerate allopatric speciation through vicariance, although isolated small populations may also face higher extinction risks1,3. The effects of habitat complexity on regional diversification are worthy of further investigation. Indeed, in the marine realm, dispersal (both positive and negative) as the dominant process may overwhelm the effects of isolation at a regional scale, for which the physical barriers are often permeable and relatively sparse30,31,32. Connectivity among marine basins may be substantially restricted because of a dynamic mosaic of ever-changing geographic and oceanographic conditions within the IAA, but not strictly or permanently blocked, and thus undermines the effectiveness of conventional allopatry. Instead, our findings may imply the importance of sympatric speciation, given the positive effect of habitat size but no effect of habitat complexity31,33. In large, continuous shelf habitats across wide environmental gradients, such as the IAA, diversification may occur along ecological partitions (that is, intense competition invokes finer subdivision and niche specialization) in addition to geographic partitions25,31,32,33. Evidence of sympatric speciation is prevalent among marine clades of all different dispersal abilities and life histories, ranging from fishes, to corals, to gastropods and to ostracods31,33,34, which potentially highlights the importance of ecological factors in marine diversification. Collectively, our MBD analysis partly supports the hopping hotspots model that proposes tectonic activity as the principal forcer of biodiversity hotspot by creating larger and more complex shallow-marine habitats1,9. We instead suggest that suitable habitat in terms of size but not complexity is important for the generation of enormous IAA diversity throughout the Cenozoic. However, note that the habitat estimations available now are based on global palaeogeographic reconstructions, so some uncertainty remains about the accuracy of such an inference. Other than the habitat factors, global temperature and sea level do not significantly correlate with diversity dynamics in the entire time frame analysis (G parameters overlapping with zero; Fig. 2a and Supplementary Table 2). This seemingly indicates that not all tectonic, eustatic, climatic, oceanographic and geomorphological processes1 are as critical as previously thought in fostering a biodiversity hotspot, or their short-term effects may be masked across a long-time frame (see the next section).
After establishing the general long-term drivers of the IAA’s diversification dynamics, we then scrutinized how their effects may vary over time across different climate regimes35. A time-stratified MBD analysis revealed further details for the warm phase (23.04–13.9 Ma), cooling phase (13.9–5.33 Ma) and cold phase (5.33–0 Ma) of the Neogene–Quaternary interval (Fig. 2b–d and Supplementary Tables 3–5). The results for the cold phase are highly concordant with those for the entire time frame, with diversity (G = -3.341, ω = 0.912) and habitat size (G = 2.445 × 10−7, ω = 0.702) negatively and positively affecting speciation, respectively (Fig. 2d and Supplementary Table 5). Higher sea level also expedited speciation during this interval (G = 0.022, ω = 0.819), probably through the species–area relationship. Large and frequent fluctuations in sea level associated with the glacial cycles may strongly affect the expansion and contraction of epicontinental seas and consequently the size of shallow-marine habitats36,37. There is no correlation between diversification dynamics and any biotic or abiotic factors for the cooling phase (Fig. 2c and Supplementary Table 4), which may indicate it as a transitional stage between two opposite climate regimes. Indeed, during the warm phase, biotic and abiotic controls on the diversification of IAA faunas occurred in very different ways from those of the cold phase (Fig. 2b and Supplementary Table 3). The strong positive effect of diversity on extinction conforms to our understanding of diversity dependency (G = 336.168, ω = 1), but its positive effect on speciation (G = 25.093, ω = 0.999) may be explained by higher availability of empty niches when there were far fewer species, as the IAA hotspot just originated in the early Miocene. Higher sea level reduced extinction as expected (G = −0.843, ω = 0.999), but it also reduced speciation, albeit more weakly (G = −0.069, ω = 0.995). A possible explanation is that Southeast Asia was still separated from the Australian and Pacific plates by deep oceans21,38, so high sea level may not contribute to form large areas of shelf habitats in the IAA. Notably, high temperature triggered both speciation (G = 0.584, ω = 0.993) and extinction (G = 5.038, ω = 0.999) during the warm phase, which may be due to the coexistence of warm-adapted and cold-adapted species. As Earth’s climate showed a long-term cooling trend across the Cenozoic39, some warm-adapted species that were resilient to or even preferred very high temperatures of the Eocene might not yet be extinct in the relatively warm early–middle Miocene40,41. They may have responded to temperature positively with more active proliferation. Cold-adapted species, by contrast, gradually rose throughout the Neogene–Quaternary as the climate further cooled4,40,42, but their vulnerability to high temperature may put them at a much higher risk of extinction during the relative warm early–middle Miocene. Indeed, recent studies suggest that tropical temperatures >25 °C may be too elevated for marine organisms owing to metabolic trade-offs driven by temperature effects on hypoxia sensitivity43,44,45. Consistent evidence for thermal stress on tropical biodiversity comes from various marine taxa (for example, coral, reef fish, reptiles and calcareous algae) that declined in diversity in the tropics and exhibited poleward range shifts during historical warm intervals45,46,47. As temperature has a much stronger impact on extinction (G = 5.04) than on speciation (G = 0.58), our analysis suggests that too high a tropical temperature in warm climates slows down diversification and hinders the growth of the hotspot. The effects of temperature are not significant for other time windows, suggesting that thermal stress may be relieved in cooler climates. We need to be aware of future extinction risks and tropical biodiversity loss exacerbated by anthropogenic warming under high greenhouse gas emission scenarios, as tropical biotas are already living close to their ecophysiological thresholds44,45.
Imprints of key biogeographic events
We have shown that the long-term diversification of the IAA was influenced by a time-sensitive series of biotic and abiotic factors. We suggest that critical shifts in tectonics, climate and oceanography may be responsible for short-term events of speciation and extinction, but also drove the biogeographic evolution of the IAA hotspot (Extended Data Fig. 5). Diversification initiated in the IAA with concurrent speciation and extinction peaks at 25 Ma, when Australia first collided with Southeast Asia21,29. The second and the largest peak in both speciation and extinction occurred at 20 Ma. It correlated in time with two major tectonic events outside and within the IAA, namely the closure of the Tethys Seaway separating the Indo-Pacific from the Mediterranean Sea and Atlantic Ocean9,48, and the closure of the Indonesian deep-water passage between Southeast Asia and Australia21. The first event enabled the delineation of the Indo-Pacific biogeographic province through the breakup of the formerly global tropical sea belt (that is, the Tethys Sea), which could naturally spur both speciation and extinction through large-scale vicariance. Also, the loss of western connectivity placed the IAA in the geographic centre of vast Indo-Pacific oceans, where the overlapping of species distributional ranges could translate to a centre of diversity (that is, mid domain effect)49. The second event established a shallow-marine connection between Southeast Asia and Australia for the first time and thus assembled a large expanse of shelf seas as suitable habitat for diversification. It could have also facilitated the convergence of peripheral faunas in the central IAA, leading to an increase in regional diversity and changes in biogeographic patterns. Then, the 16-Ma peak coincided with the Mid-Miocene Climatic Optimum40, which is in line with our MBD results indicating that both speciation and extinction rates accelerated with high temperature in a warm climate state (Fig. 2b). Similar diversification peaks at about 18 and 15 Ma were also found in Caribbean bryozoans8, which may correspond to our 20-Ma and 16-Ma events. The 12-Ma peak was apparent only in the speciation rate when the East Asian monsoon intensified50. A stronger regional environmental gradient, such as salinity through the enhanced monsoon and resulting precipitation, could provide a possible explanation. Finally, the 5-Ma speciation and extinction peaks may be related to the restriction of the Indonesian Throughflow at the Miocene–Pliocene boundary38,51, which allowed reversed faunal dispersal from the Indian Ocean to the Pacific Ocean3. In summary, abrupt transitions in Earth’s physical environments probably introduced short-term instability into the biotic system. For each of the biotic events, extinctions were overcompensated with much stronger speciation to support overall diversification, indicating the role of the IAA as a reservoir for species accumulation and a source of species origination.
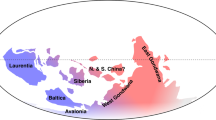
Among all palaeoenvironmental events discussed above, the restriction and final closure of the Tethys Seaway during about 25–20 Ma is of particular interest. This process governed the palaeobiogeographic shifts from the vanishing Tethys to the emerging IAA, which are considered an important factor in shaping the latter hotspot by the hopping hotspots model9. Here we investigated how the migration of the Tethyan relicts may leave long-lasting implications on the diversity and biogeographic history of the IAA hotspot. We focused on the five most diverse genera of the Tethyan group (originated in the broad Tethyan region during the Palaeogene52,53), cosmopolitan group (exhibited global distributions during the Cenozoic54,55) and IAA group (originated in the central Indo-Pacific region during the Neogene54,55,56), respectively, and compared their proliferation across the Cenozoic (Fig. 3 and Extended Data Fig. 6). From the Eocene to early Miocene, the Tethyan genera exhibited a strong increase in diversity (2 to 14 species) and gained dominance over the other groups. During the middle–late Miocene, all three groups showed substantial proliferation with the cosmopolitan genera being more diverse than the Tethyan and IAA genera (75 compared with 46 and 20, respectively). The long-term trend of diversification proceeded to the Pliocene for the cosmopolitan and IAA groups with remarkable increases in diversity (156 and 28, respectively), whereas that of the Tethyan genera declined (42 species). Finally, slight Pleistocene drops in diversity probably represented sampling artefacts instead of true extinction, at least for the cosmopolitan and IAA groups, and the cessation of diversification corresponded to the maturation of the IAA hotspot. The evolution of three biogeographic groups closely mirrors that of the whole ostracod assemblage, as the average per-genus diversity reached a value of 1.29, 2.62 and 4.34 in the Eocene, Miocene and Pliocene, despite a slight decrease to 3.9 in the Pleistocene, indicating a pronounced diversification at species level throughout the Cenozoic (Fig. 4). The waxing and waning of these three groups clearly reflect a changing biogeographic affinity of the IAA with other regions. Before the final closure of the Tethys Seaway, the Tethyan ancestors were able to reach the IAA after a long-distance dispersal. They persisted and proliferated in the IAA even after the seaway closed, and thereby supported the early-stage development of the IAA hotspot. Since the middle Miocene, the IAA situated at the centre of the vast Indo-Pacific domain with high connectivity and dispersal may be more prone to accommodate markedly radiating cosmopolitan taxa from the Indian and Pacific oceans, which then outcompeted the earlier occupants of the Tethyan genera. In addition, the endemic IAA genera were comparatively less diverse, possibly because of their youngest age; nevertheless, they also experienced a prominent diversification since the late Miocene. Their success may indicate the importance of tropical hotspots in generating and exporting biodiversity within a relatively short geological time. We conclude that the diversification of the IAA hotspot was accompanied by profound biogeographic changes, as the Tethyan taxa were gradually replaced by cosmopolitan and IAA taxa during the Neogene–Quaternary. Tethyan faunal elements from older, vanished hotspots did become evolutionary fuel for the emergence of the IAA, as proposed by the hopping hotspots model, but such a unique palaeobiogeographic shift was not the sole mechanism that contributed to tropical diversification. In fact, the stepwise collision of Australia with Southeast Asia and the switching on and off of the Indonesian Throughflow could both be important processes that facilitated the convergence, accumulation and eventually proliferation of taxa in the central IAA.
Stack bars show the sum of per-genus diversity of the five most diverse cosmopolitan, Tethyan and IAA genera during each interval of the Cenozoic. Eoc, Eocene; Oligo, Oligocene; earlyMio, early Miocene; midMio, middle Miocene; lateMio, late Miocene; Plio, Pliocene; Pleisto, Pleistocene.
The five most diverse genera of the cosmopolitan, Tethyan and IAA groups are shown in the corresponding colour used in Fig. 3. The error bar shows standard deviation (if the lower limit is below zero, the minimum value is used instead), with the black circles indicating mean per-genus diversity of all ostracod genera.
Another possible ecological explanation for the success of cosmopolitan and IAA genera is their adaptation to the glacio-eustatic sea-level fluctuations of the Plio-Pleistocene. During this interval, ostracod taxa with strong depth-preference conservatism may have been forced to repeatedly migrate downslope and upslope to follow successive cycles of large-amplitude sea-level changes13. Most Tethyan genera were found exclusively in shallow-marine environments and had relatively narrow depth niches52,53. They may be less adaptable to large-scale sea-level changes than some deeper-water cosmopolitan genera (for example, Cytheropteron, Krithe and Argilloecia), which thrived in a wider range of depths from the continental shelf to the continental slope13. To sum up, the Plio-Pleistocene decline of Tethyan genera is probably due to a combination of long-term palaeobiogeographic reorganization and short-term palaeoceanographic changes.
Our results suggest that the increase in habitat size and immunity to major extinction events during the Cenozoic allowed for strong, uninterrupted long-term diversification that reached an asymptote in the IAA, making it the current global centre of biodiversity. The relief of thermal stress as Earth’s climate transformed from a warm to a cold state was also essential for this process. It took tens of millions of years for the IAA hotspot to fully develop in an ideal environment in terms of climate, habitat and biogeographic connectivity, without, by chance, a major extinction event. As we know, the opposite is now happening, with anthropogenic warming57, habitat destruction58 and the sixth mass extinction44,59. All may effectively undermine the IAA hotspot and impair its function as a cradle and museum of tropical biodiversity. Our palaeobiological results highlight the slow evolution, in contrast to the ongoing decadal degradation, of the tropical hotspot as one of the most remarkable biodiversity and biogeographic patterns, and urge conservation efforts.
Methods
We first summarize how we obtained the Cenozoic IAA fossil dataset, integrating our original data with published data. We then detail the application of birth–death models to reconstruct the IAA’s diversity history, and to uncover its controlling factors. Finally, we describe the raw analyses of the fossil dataset from a biogeographic perspective.
Sediment samples, sample processing and ostracod identification
A total of 188 Neogene and Quaternary outcrop sediment samples were collected from the islands of Luzon, Cebu, Negros, Panay, Leyte, Bohol, Mindanao and Samar, Philippines, in addition to the islands of Java and Kalimantan, Indonesia (Extended Data Fig. 1). The ages of all sediment samples were determined from planktic foraminiferal and calcareous nannofossil biochronology following the geological timescale in refs. 60,61 and Southeast Asia stratigraphy62. We used a standard sample processing method for microfossil ostracod research; that is, outcrop samples were wet-sieved through a 63-μm mesh sieve, oven dried and then dry-sieved through a 150-μm mesh sieve13. We retained the >150 μm size fraction, from which adults and late moulting juveniles of ostracods were picked. Specimens <150 μm are usually early moulting juveniles for most genera, which are too delicate and too small to be preserved and identified. Therefore, the effect of mesh size on species composition and diversity measurements is likely to be negligible13. Samples with too many ostracod specimens were divided using a sample splitter. All ostracods in a sample or a split aliquot were picked up and sorted on a micropalaeontological cardboard slide. Most ostracods were identified to the species level. Taxon counts considered one valve or articulated carapace as one specimen, which is the standard method for counting ostracods13.
Data integration
We integrated published Cenozoic IAA data into this study after rigorous taxonomic harmonization, including data from Leyte Island, Philippines, in addition to the islands of Java and Kalimantan, Indonesia (Extended Data Fig. 1)13,63. Most of the original and published samples are in excellent ostracod preservation, with a few middle-Miocene samples in moderate preservation. Even Eocene specimens have glassy, translucent shells, and thus it is unlikely that preservation bias substantially affects our biodiversity results, as discussed in detail previously13. The depositional environments of the published sites vary from the continental shelf to the upper continental slope, all under fully marine conditions13,63. Consistently, the fossil ostracod faunas of our original IAA samples show the same diversity of depth habitats, ranging from shallow marine to the shallowest deep sea (upper bathyal). With the compilation of published sources with original data, this study addresses biotic changes in primarily continental shelf benthic ecosystems across the Cenozoic. Spatial coverage of all aggregated samples is reasonably good for representing the IAA region as a whole, especially within the Philippines and central Indonesia where modern diversity is known to be the highest10,22 (Extended Data Fig. 4).
Estimating speciation and extinction dynamics
The application of the birth–death model deciphered the macroevolutionary dynamics of the IAA hotspot by estimating historical variations in speciation and extinction rates across the Cenozoic while incorporating sampling biases and uncertainties associated with the age of each fossil occurrence. We carried out analyses of the raw ostracod fossil dataset based on the Bayesian framework as implemented in the PyRate 3.0 program64,65. The robustness of PyRate was rigorously tested against a range of potential biases, including violations of the sampling assumptions, variable preservation rates and incomplete taxon sampling, and it has been shown to correctly recover the dynamics of speciation and extinction in the face of sudden rate changes and mass extinction66. We analysed the datasets under time-varying birth–death models to simultaneously estimate for each clade: the parameters of the preservation process; the times of speciation (Ts) and extinction (Te) of each ostracod species; and the speciation (λ) and extinction (μ) rates and their variation through time. The PyRate approach benefits from the estimation of Ts and Te of the studied species taking into account preservation biases, and thus provides estimates of species longevity (that is, the duration or time span of a species). This reduces the issues associated with estimating speciation and extinction rates and estimating the number of species through time64,65. Based on all taxon occurrences, the preservation rate is expressed as expected occurrences per taxon per million years (Myr) and contributes to the inference of the individual origination and extinction times of each taxon. We first determined the best-fit preservation process for the data using the -PPmodeltest option, which compares the homogeneous Poisson process, the non-homogeneous Poisson process and the time-variable Poisson process. We carried out the recently enhanced version through the reversible-jump Markov chain Monte Carlo (MCMC) algorithm (-A 4 option), which gives more accurate estimates of speciation and extinction rates compared with traditional approaches including boundary-crossers and three-timers66.
We ran PyRate for 50 million MCMC generations for the birth–death with constrained shifts (BDCS) model67. Given the high resolution of the dataset, we defined time bins of 2 Myr (-fixShift option) across the Cenozoic. All analyses were set with a non-homogeneous Poisson process of preservation (determined as the best model), and accounted for varying preservation rates across taxa using the Gamma model (-mG option); that is, with Γ-distributed rate heterogeneity. We monitored chain mixing and effective sample sizes by examining the log files in Tracer 1.7.168 after excluding the first 10% of the samples as a burn-in period. We then combined the posterior estimates of the speciation and extinction rates across all replicates to generate rates-through-time plots (speciation, extinction and net diversification). Rates of two adjacent intervals (2 Myr) were considered significantly different when the mean of one lay outside the 95% credibility intervals of the other, and vice versa. We replicated the analyses on ten randomized datasets and calculated estimates of times of speciation and times of extinction (Ts–Te data) as the mean of the posterior samples from each replicate. Thus, we obtained ten posterior estimates of the times of speciation and extinction for all species and used them as input data in all of the subsequent analyses (see below), which therefore focused exclusively on the estimation of the birth–death parameters (that is, without remodelling preservation and re-estimating times of speciation and extinction). This procedure markedly reduces the computational burden, while allowing us to account for the preservation process and the uncertainties associated with the fossil ages.
Estimating palaeoenvironment-dependent diversification
We then used the estimated times of speciation and extinction (Ts–Te data) of all species to identify putative causal mechanisms for the diversification of shallow-marine taxa in the IAA hotspot. We simultaneously examined the correlation of a set of palaeoenvironmental variables with speciation and extinction rates throughout the Cenozoic. We focused on temperature, sea level, habitat size and complexity (representing the effects of tectonic movements) as four abiotic factors and diversity dependency as one biotic factor, all of which have been linked to biodiversity changes in marine invertebrates and have been suggested to play an important role in the development of hotspots2,3,36,69,70. Trends in global temperature change are typically based on oxygen isotope ratios (δ18O) in benthic foraminifera and a smoothed Cenozoic sea surface temperature record is derived from ref. 71. Similarly, fluctuations in the Cenozoic global mean sea level are derived from benthic foraminiferal δ18O and Mg/Ca records that document the evolution from the Eocene ice-free conditions to Quaternary bipolar ice sheets causing eustatic variations72. Regional changes in habitat size and complexity are measured as total shelf area and shoreline length, respectively, using palaeogeographic reconstructions of palaeo-coastlines and flooded continental shelf distributions in the IAA for the past 50 Myr3,73. Shelf area is delimited between the reconstructed maximum transgression coastline and continental margin, and shoreline length is calculated using the ‘yardstick’ method73,74.
After analysing the long-term drivers of the diversification dynamics, we then estimated whether palaeoenvironmental changes may have varying effects through time in the IAA. As there was differential diversification dynamics over time, it is likely that palaeoenvironmental changes had different impacts under different climatic regimes. To study such effects, we carried out a time-stratified MBD analyses assuming different diversification dynamics and correlations to each studied factor during the warm phase (23.04–13.9 Ma), cooling phase (13.9–5.33 Ma) and cold phase (5.33–0 Ma) of the Neogene–Quaternary interval, as segmented by the Middle Miocene Climate Transition and the Pliocene–Pleistocene transition39. Such a division scheme reflects the global climate evolution across the Neogene–Quaternary.
We used the MBD model to assess to what extent biotic and abiotic factors can explain temporal variation in speciation and extinction rates75. Under the MBD model, speciation and extinction rates can change through time but equally across all lineages, as in the BDCS model, through correlations with multiple time-continuous variables, and the strengths and signs (positive or negative) of the correlations are jointly estimated for each variable. We applied exponential correlations between rates and environmental variables. The MBD model also makes the hypothesis that such correlations are time continuous66,76. The correlation parameters (G) can take negative values indicating negative correlation, or positive values for positive correlations. When their value is estimated to be approximately zero, there is no correlation. A MCMC algorithm jointly estimates the baseline speciation (λ0) and extinction (µ0) rates and all correlation parameters (Gλ and Gµ) using a horseshoe prior to control for over-parameterization and for the potential effects of multiple testing75. The horseshoe prior provides an approach to distinguish correlation parameters that should be treated as noise (and therefore are around 0) from those that are significantly different from 0 and represent true signal.
We ran the MBD model using 40 million MCMC iterations and sampling every 40,000 to approximate the posterior distribution of all parameters (λ0, µ0, five Gλ, five Gµ and the shrinkage weights of each correlation parameter, ωG). We summarized the results of the MBD analyses by calculating the posterior mean and 95% credibility interval of all correlation parameters and the mean of the respective shrinkage weights (ω) across ten replicates, as well as the mean and 95% credibility interval of the baseline speciation and extinction rates. A given environmental variable must have ω > 0.5 and 95% credibility interval for G ≠ 0 to be considered as having a significant effect on rate variations. Regarding the biological meaning of the parameters estimated with temperature, for instance, the estimation of a positive Gλ would indicate that higher temperatures increase speciation rates, whereas a negative Gλ would indicate that higher temperatures decrease speciation rates. The same rationale applies to the extinction rate but with the parameter Gμ quantifying the correlation between changes in extinction rates and this variable. Let us imagine the positive effect of temperature on speciation given by Gλ = 0.05. This result means that speciation and temperature correlate positively such that speciation increased by 5% as global temperatures increased at every time step (here, every 0.1 million years).
Sensitivity tests
Regarding the estimation of the speciation and extinction dynamics, we noted that potential sampling bias could be caused by the lack of the Oligocene data and the binning of the fossil record. To explore the sensitivity of the speciation and extinction estimates, we repeated all of the BDCS analyses for the Neogene–Quaternary alone with time bins of 2 Myr, for the entire Cenozoic with time bins of 5 Myr and for the Neogene–Quaternary alone with time bins of 5 Myr. All of these analyses were parameterized following the main analysis. All results corroborate a reasonably robust and unambiguous pattern of strong and continuous diversification throughout the studied intervals (Extended Data Figs. 7–9).
For disentangling the palaeoenvironmental controls on diversification dynamics, we reasoned that the use of global temperature in a regional analysis of the IAA could pose certain limitations on our modelling, together with the Oligocene sampling gap. We ran two sensitivity MBD analyses using the tropical temperature record from ref. 77 for the Cenozoic and for the Neogene–Quaternary instead of the global temperature. With all other parameters unchanged, the results remain consistent with those from the main analysis, still showing that diversity dependence and habitat size are two determinants of speciation (Supplementary Tables 6 and 7).
Raw analyses of the fossil dataset
We also carried out rarefaction, E(S200), the expected number of species if 200 specimens were sampled to calculate species α-diversity, taking into account the sample size artefact; thus, only samples with ≥200 individuals were used to calculate diversity. We illustrated the stratigraphic ranges for all ostracod taxa by connecting the earliest and latest occurrence of every taxon. In addition, we counted diversity per genus as the number of species in a genus and calculated the mean diversity per genus of all genera occurring within each geological interval. Among all genera that were relatively abundant and speciose throughout the Cenozoic in the IAA, we identified typical Tethyan genera that originated in the broad Tethys biogeographic area during the Palaeogene (that is, Neomonoceratina in the Palaeocene and Stigmatocythere, Pokornyella, Keijella and Tenedocythere in the Eocene)52,53,78,79,80; cosmopolitan genera that had a broad geographical distribution across the Cenozoic (Cytheropteron, Krithe, Argilloecia, Loxoconcha and Xestoleberis)54,55; and IAA genera that first appeared in the central Indo-Pacific during the Neogene (that is, Pistocythereis, Lankacythere, Atjehella, Hemikrithe and Hemicytheridea in the Miocene54,55,56,81,82). Finally, we compared the trends of their respective diversity changes per genus throughout the Cenozoic. All of the above analyses were carried out in the R programming language (version 4.3.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available via Figshare at https://doi.org/10.6084/m9.figshare.25395871.v2 (ref. 83). Source data are provided with this paper.
Code availability
The codes supporting the findings of this study are available via Figshare at https://doi.org/10.6084/m9.figshare.25395871.v2 (ref. 83).
References
Bellwood, D. R., Renema, W. & Rosen, B. R. in Biotic Evolution and Environmental Change in Southeast Asia Vol. 82 (eds Gower, D. J. et al.) 216–245 (Cambridge Univ. Press, 2012).
Tittensor, D. P. et al. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 (2010).
Yasuhara, M. et al. Hotspots of Cenozoic tropical marine biodiversity. Oceanogr. Mar. Biol. Annu. Rev. 60, 243–300 (2022).
Ezard, T. H., Aze, T., Pearson, P. N. & Purvis, A. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351 (2011).
Lowery, C. M., Bown, P. R., Fraass, A. J. & Hull, P. M. Ecological response of plankton to environmental change: thresholds for extinction. Annu. Rev. Earth Planet. Sci. 48, 403–429 (2020).
Renema, W. in Biogeography, Time, and Place: Distributions, Barriers, and Islands (ed. Renema, W.) 179–215 (Springer, 2007).
Johnson, K. G., Renema, W., Rosen, B. R. & Santodomingo, N. Old data for old questions: what can the historical collections really tell us about the Neogene origins of reef-coral diversity in the Coral Triangle? Palaios 30, 94–108 (2015).
Di Martino, E., Jackson, J. B., Taylor, P. D. & Johnson, K. G. Differences in extinction rates drove modern biogeographic patterns of tropical marine biodiversity. Sci. Adv. 4, eaaq1508 (2018).
Renema, W. et al. Hopping hotspots: global shifts in marine biodiversity. Science 321, 654–657 (2008).
Hoeksema, B. W. in Biogeography, Time, and Place: Distributions, Barriers, and Islands (ed. Renema, W.) 117–178 (Springer, 2007).
Bellwood, D. R., Goatley, C. H. & Bellwood, O. The evolution of fishes and corals on reefs: form, function and interdependence. Biol. Rev. 92, 878–901 (2017).
Cowman, P. F. & Bellwood, D. R. The historical biogeography of coral reef fishes: global patterns of origination and dispersal. J. Biogeogr. 40, 209–224 (2013).
Yasuhara, M. et al. Cenozoic dynamics of shallow‐marine biodiversity in the Western Pacific. J. Biogeogr. 44, 567–578 (2017).
Mihaljević, M., Korpanty, C., Renema, W., Welsh, K. & Pandolfi, J. M. Identifying patterns and drivers of coral diversity in the Central Indo-Pacific marine biodiversity hotspot. Paleobiology 43, 343–364 (2017).
Harzhauser, M., Mandic, O., Piller, W. E., Reuter, M. & Kroh, A. Tracing back the origin of the Indo‐Pacific mollusc fauna: basal Tridacninae from the Oligocene and Miocene of the sultanate of Oman. Palaeontology 51, 199–213 (2008).
Yasuhara, M., Tittensor, D. P., Hillebrand, H. & Worm, B. Combining marine macroecology and palaeoecology in understanding biodiversity: microfossils as a model. Biol. Rev. 92, 199–215 (2017).
Chiu, W. T. R. et al. Marine latitudinal diversity gradients, niche conservatism and out of the tropics and Arctic: climatic sensitivity of small organisms. J. Biogeogr. 47, 817–828 (2020).
Jöst, A. B. et al. North Atlantic Gateway: test bed of deep‐sea macroecological patterns. J. Biogeogr. 46, 2056–2066 (2019).
Hong, Y. et al. Benthic ostracod diversity and biogeography in an urbanized seascape. Mar. Micropaleontol. 174, 102067 (2022).
Leray, M. & Knowlton, N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl Acad. Sci. USA 112, 2076–2081 (2015).
Hall, R. Southeast Asia’s changing palaeogeography. Blumea 54, 148–161 (2009).
Sanciangco, J. C., Carpenter, K. E., Etnoyer, P. J. & Moretzsohn, F. Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS ONE 8, e56245 (2013).
O’Dea, A. et al. Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl Acad. Sci. USA 104, 5501–5506 (2007).
Morlon, H. Phylogenetic approaches for studying diversification. Ecol. Lett. 17, 508–525 (2014).
Cornell, H. V. Is regional species diversity bounded or unbounded? Biol. Rev. 88, 140–165 (2013).
Rabosky, D. L. Diversity-dependence, ecological speciation, and the role of competition in macroevolution. Annu. Rev. Ecol. Evol. Syst. 44, 481–502 (2013).
Kisel, Y., McInnes, L., Toomey, N. H. & Orme, C. D. L. How diversification rates and diversity limits combine to create large-scale species–area relationships. Philos. Trans. R. Soc. B 366, 2514–2525 (2011).
Losos, J. B. & Schluter, D. Analysis of an evolutionary species–area relationship. Nature 408, 847–850 (2000).
Hall, R., Cottam, M. A. & Wilson, M. E. The SE Asian Gateway: History and Tectonics of the Australia-Asia Collision 1–6 (Geological Society, London, 2011).
Wood, S., Paris, C., Ridgwell, A. & Hendy, E. Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecol. Biogeogr. 23, 1–11 (2014).
Bowen, B. W., Rocha, L. A., Toonen, R. J. & Karl, S. A. The origins of tropical marine biodiversity. Trends Ecol. Evol. 28, 359–366 (2013).
Norris, R. D. Pelagic species diversity, biogeography, and evolution. Paleobiology 26, 236–258 (2000).
Rocha, L. A., Robertson, D. R., Roman, J. & Bowen, B. W. Ecological speciation in tropical reef fishes. Philos. Trans. R. Soc. B 272, 573–579 (2005).
Cronin, T. M. & Schmidt, N. Evolution and biogeography of Orionina in the Atlantic, Pacific and Caribbean: evolution and speciation in Ostracoda II. Dev. Palaeontol. Stratigr. 11, 927–938 (1988).
Neubauer, T. A. et al. Drivers of diversification in freshwater gastropods vary over deep time. Philos. Trans. R. Soc. B 289, 20212057 (2022).
Tennant, J. P., Mannion, P. D. & Upchurch, P. Sea level regulated tetrapod diversity dynamics through the Jurassic/Cretaceous interval. Nat. Commun. 7, 12737 (2016).
Ludt, W. B. & Rocha, L. A. Shifting seas: the impacts of Pleistocene sea‐level fluctuations on the evolution of tropical marine taxa. J. Biogeogr. 42, 25–38 (2015).
Kuhnt, W., Holbourn, A., Hall, R., Zuvela, M. & Käse, R. in Continent-Ocean Interactions within East Asian Marginal Seas Vol. 149 (eds Clift, P. et al.) 299–320 (American Geophysical Union, 2004).
Westerhold, T. et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383–1387 (2020).
Steinthorsdottir, M. et al. The Miocene: the future of the past. Paleoceanogr. Paleoclimatol. 36, e2020PA004037 (2021).
Mathes, G. H., van Dijk, J., Kiessling, W. & Steinbauer, M. J. Extinction risk controlled by interaction of long-term and short-term climate change. Nat. Ecol. Evol. 5, 304–310 (2021).
De Blasio, F. V., Liow, L. H., Schweder, T. & De Blasio, B. F. A model for global diversity in response to temperature change over geological time scales, with reference to planktic organisms. J. Theor. Biol. 365, 445–456 (2015).
Yasuhara, M. et al. Past and future decline of tropical pelagic biodiversity. Proc. Natl Acad. Sci. USA 117, 12891–12896 (2020).
Penn, J. L. & Deutsch, C. Avoiding ocean mass extinction from climate warming. Science 376, 524–526 (2022).
Costello, M. J. et al. The universal evolutionary and ecological significance of 20 °C. Front. Biogeogr. 15, e61673 (2023).
Kiessling, W., Simpson, C., Beck, B., Mewis, H. & Pandolfi, J. M. Equatorial decline of reef corals during the last Pleistocene interglacial. Proc. Natl Acad. Sci. USA 109, 21378–21383 (2012).
Sun, Y. et al. Lethally hot temperatures during the Early Triassic greenhouse. Science 338, 366–370 (2012).
Hou, Z. & Li, S. Tethyan changes shaped aquatic diversification. Biol. Rev. 93, 874–896 (2018).
Bellwood, D., Hughes, T., Connolly, S. & Tanner, J. Environmental and geometric constraints on Indo‐Pacific coral reef biodiversity. Ecol. Lett. 8, 643–651 (2005).
Tada, R., Zheng, H. & Clift, P. D. Evolution and variability of the Asian monsoon and its potential linkage with uplift of the Himalaya and Tibetan Plateau. Prog. Earth Planet. Sci. 3, 4 (2016).
Gallagher, S. J. et al. Neogene history of the West Pacific warm pool, Kuroshio and Leeuwin currents. Paleoceanography 24, PA1206 (2009).
Yamaguchi, T. & Kamiya, T. Eocene ostracodes from Hahajima Island of the Ogasawara (Bonin) Islands, northwestern Pacific, and their paleobiogeographic significance in the West Pacific. J. Paleontol. 83, 219–237 (2009).
Yasuhara, M. et al. Eocene shallow-marine ostracods from Madagascar: southern end of the Tethys? J. Syst. Paleontol. 17, 705–757 (2019).
Benson, R. et al. Treatise on Invertebrate Paleontology: Arthropoda 3: Crustacea, Ostracoda. (Geological Society of America, 1961).
van Morkhoven, F. P. C. M. Post-Palaeozoic Ostracoda: Their Morphology, Taxonomy and Economic Use: Vol. I: General (Elsevier, 1962).
Kingma, J. T. Contributions to the Knowledge of the Young-Caenozoic Ostracoda from the Malayan Region (Univ. Utrecht, 1948).
O’Neill, B. C. et al. IPCC reasons for concern regarding climate change risks. Nat. Clim. Change 7, 28–37 (2017).
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 (2015).
Finnegan, S. et al. Paleontological baselines for evaluating extinction risk in the modern oceans. Science 348, 567–570 (2015).
Speijer, R., Pälike, H., Hollis, C., Hooker, J. & Ogg, J. in Geologic Time Scale 2020 (eds Gradstein, F. M. et al.) 1087–1140 (Elsevier, 2020).
Raffi, I. et al. in Geologic Time Scale 2020 (eds Gradstein, F. M. et al.) 1141–1215 (Elsevier, 2020).
Aurelio, M. & Peña, R. Geology of the Philippines 2nd edn (Mines and Geosciences Bureau, 2010).
Shin, C. P. et al. Neogene marine ostracod diversity and faunal composition in Java, Indonesia: Indo-Australian Archipelago biodiversity hotspot and the Pliocene diversity jump. J. Crustac. Biol. 39, 244–252 (2019).
Silvestro, D., Salamin, N. & Schnitzler, J. PyRate: a new program to estimate speciation and extinction rates from incomplete fossil data. Methods Ecol. Evol. 5, 1126–1131 (2014).
Silvestro, D., Salamin, N., Antonelli, A. & Meyer, X. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology 45, 546–570 (2019).
Condamine, F. L., Guinot, G., Benton, M. J. & Currie, P. J. Dinosaur biodiversity declined well before the asteroid impact, influenced by ecological and environmental pressures. Nat. Commun. 12, 3833 (2021).
Silvestro, D., Antonelli, A., Salamin, N. & Quental, T. B. The role of clade competition in the diversification of North American canids. Proc. Natl Acad. Sci. USA 112, 8684–8689 (2015).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Mayhew, P. J., Bell, M. A., Benton, T. G. & McGowan, A. J. Biodiversity tracks temperature over time. Proc. Natl Acad. Sci. USA 109, 15141–15145 (2012).
Zaffos, A., Finnegan, S. & Peters, S. E. Plate tectonic regulation of global marine animal diversity. Proc. Natl Acad. Sci. USA 114, 5653–5658 (2017).
Boschman, L. M. & Condamine, F. L. Mountain radiations are not only rapid and recent: ancient diversification of South American frog and lizard families related to Paleogene Andean orogeny and Cenozoic climate variations. Glob. Planet. Change 208, 103704 (2022).
Miller, K. G. et al. Cenozoic sea-level and cryospheric evolution from deep-sea geochemical and continental margin records. Sci. Adv. 6, eaaz1346 (2020).
Kocsis, Á. T. & Scotese, C. R. Mapping paleocoastlines and continental flooding during the Phanerozoic. Earth Sci. Rev. 213, 103463 (2021).
O’Sullivan, D. & Unwin, D. Geographic Information Analysis (Wiley, 2003).
Lehtonen, S. et al. Environmentally driven extinction and opportunistic origination explain fern diversification patterns. Sci. Rep. 7, 4831 (2017).
Condamine, F. L., Romieu, J. & Guinot, G. Climate cooling and clade competition likely drove the decline of lamniform sharks. Proc. Natl Acad. Sci. USA 116, 20584–20590 (2019).
Scotese, C. R., Song, H., Mills, B. J. & van der Meer, D. G. Phanerozoic paleotemperatures: the earth’s changing climate during the last 540 million years. Earth Sci. Rev. 215, 103503 (2021).
Guernet, C., Bourdillon, C. & Roger, J. Palaeogene ostracods of Dohofar (Oman) stratigraphical and paleogeographical implication. Rev. Micropaleontol. 34, 297–311 (1991).
Yasuhara, M. et al. Early Miocene marine ostracodes from southwestern India: implications for their biogeography and the closure of the Tethyan Seaway. J. Paleontol. 94, 1–36 (2020).
Tanaka, G. et al. First discovery of Eocene coastal-estuarine ostracods from Japan, with the geological history of the migration of estuarine genera in the Far East. Geol. Mag. 155, 1742–1760 (2018).
Whatley, R. & Quanhong, Z. Recent ostracoda of the Malacca Straits. Part. II. (Continuation). Revista Esp. Micropaleontol. 20, 5–37 (1988).
Keij, A. Review of the Indo-westpacific Neogene to Holocene ostracode genus Atjehella. Proc. Kon. Ned. Akad. Wetensch. 82, 449–464 (1979).
Condamine, F., Tian, S. Y. & Yasuhara, M. Cenozoic history of the tropical marine biodiversity hotspot. figshare https://doi.org/10.6084/m9.figshare.25395871.v2 (2024).
Acknowledgements
We thank R. P. P. Wong and M. G. Y. Lo for continuous support; H. Hayashi for help with biostratigraphy; A. Zhao for help with sampling processing; J. Zhang for help with taxonomic identification; and L. Cotton, A. Lam and A. Woodhouse for comments. This work was partly supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (project codes: RFS2223-7S02, HKU 17300821, HKU 17300720, HKU 17302518, C7013-19G and G-HKU709/21), the Small Equipment Grant of the University of Hong Kong, the Seed Funding Programme for Basic Research of the University of Hong Kong (project codes: 202111159167, 202011159122 and 201811159076), the Faculty of Science RAE Improvement Fund of the University of Hong Kong, the Seed Funding of the HKU-TCL Joint Research Centre for Artificial Intelligence of the University of Hong Kong, and the State Key Laboratory of Marine Pollution Seed Collaborative Research Fund (SKLMP/SCRF/0031; to M.Y.). S.Y.T. was supported by the Humboldt Research Fellowship.
Author information
Authors and Affiliations
Contributions
S.Y.T. and M.Y. designed research; S.Y.T., M.Y., T.K., A.G.S.F., Y.M.A., H.P., T.I., H.I., C.P.S. and W.R. carried out research; S.Y.T., F.L.C., H.-H.M.H. and M.Y. analysed data; and S.Y.T., M.Y. and F.L.C. wrote the paper. S.Y.T. created the silhouettes of each ostracod genus in Fig. 3.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Laura Cotton, Adriane Lam, Adam Woodhouse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
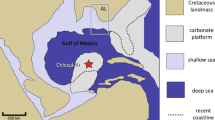
Extended Data Fig. 1 Map of the Indo-Australian Archipelago showing sampling location, with a magnification of the Philippine islands.
The name of each sampling island is labelled in red.
Extended Data Fig. 2 Ostracod diversity trajectory over the Cenozoic in the IAA, based on species richness from rarefaction E(S200) for sediment samples (n = 111).
Box hinges represent the first and third quartiles with the center as median, and whiskers extend to 1.5× the interquartile range (IQR) from the first and third quartiles. Minima and maxima beyond the whiskers are individually plotted outlying points. Eoc: Eocene; Oligo: Oligocene; earlyMio: early Miocene; midMio: middle Miocene; lateMio: late Miocene; Plio: Pliocene; Pleisto: Pleistocene.
Extended Data Fig. 3 Stratigraphic ranges for all 874 ostracod taxa in the IAA in this study.
The ostracod taxa (780 species, and 94 genus level entries) are classified by their earliest stratigraphic occurrence, i.e., Eocene, Oligocene, early Miocene, middle Miocene, late Miocene, Pliocene, and Pleistocene taxa. The range for each taxon is computed by connecting the minimum and maximum ages of the taxon occurrence by a vertical line, and individual taxa occurrences are indicated by dots. Within each interval, the taxa are ranked by the age of first occurrence. Dashed horizontal lines indicate chronostratigraphic boundary. Abbreviations as in Extended Data Fig. 2.
Extended Data Fig. 4 Diversity map showing species richness from rarefaction E(S200) in each geological interval.
Eocene: 56-33.9 Ma; Oligocene: 33.9-23.04 Ma; early Miocene: 23.04-15.99 Ma; middle Miocene: 15.99-11.65 Ma; late Miocene: 11.65-5.33 Ma; Pliocene: 5.33-2.58 Ma; Pleistocene: 2.58-0.01 Ma.
Extended Data Fig. 5 Schematic diagram showing the short-term biotic events in relation to key changes in tectonics, climate and oceanography.
25 Ma: first collision of Australia with Southeast Asia; 20 Ma: closure of the Tethys Seaway and closure of the Indonesian deep-water passage; 16 Ma: Mid-Miocene Climate Optimum; 12 Ma: intensification of the East Asian Monsoon; 5 Ma: restriction of the Indonesian Throughflow. Blue and orange shaded areas in the maps indicate continental shelf. Paleogeographic maps modified from Kocsis and Scotese73.
Extended Data Fig. 6 Species-level diversification of the five most diverse cosmopolitan, Tethyan, and IAA genera across the Cenozoic.
Dashed horizontal lines indicate chronostratigraphic boundary.
Extended Data Fig. 7 Estimation of regional diversity dynamics in the IAA across the Neogene-Quaternary with 2 Myr bin.
Bayesian inferences of (a) speciation rates per lineage, (b) extinction rates per lineage, (c) net diversification rates as the difference between speciation and extinction rates (rates below 0 indicate declining diversity), and (d) the changes in species richness. Solid lines indicate mean posterior rates, and the shaded areas show the 95% confidence interval. Early Miocene, late Miocene, Pleistocene are shaded. Vertical dotted lines indicate epoch boundaries.
Extended Data Fig. 8 Estimation of regional diversity dynamics in the IAA across the Cenozoic with 5 Myr bin.
Bayesian inferences of (a) speciation rates per lineage, (b) extinction rates per lineage, (c) net diversification rates as the difference between speciation and extinction rates (rates below 0 indicate declining diversity), and (d) the changes in species richness. Solid lines indicate mean posterior rates, and the shaded areas show the 95% confidence interval. Early Oligocene, early Miocene, late Miocene, Pleistocene are shaded. Vertical dotted lines indicate epoch boundaries.
Extended Data Fig. 9 Estimation of regional diversity dynamics in the IAA across the Neogene-Quaternary with 5 Myr bin.
Bayesian inferences of (a) speciation rates per lineage, (b) extinction rates per lineage, (c) net diversification rates as the difference between speciation and extinction rates (rates below 0 indicate declining diversity), and (d) the changes in species richness. Solid lines indicate mean posterior rates, and the shaded areas show the 95% confidence interval. Early Miocene, late Miocene, Pleistocene are shaded. Vertical dotted lines indicate epoch boundaries.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, S.Y., Yasuhara, M., Condamine, F.L. et al. Cenozoic history of the tropical marine biodiversity hotspot. Nature (2024). https://doi.org/10.1038/s41586-024-07617-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07617-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.