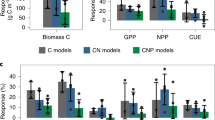
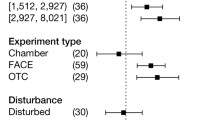
Abstract
Atmospheric carbon dioxide enrichment (eCO2) can enhance plant carbon uptake and growth1,2,3,4,5, thereby providing an important negative feedback to climate change by slowing the rate of increase of the atmospheric CO2 concentration6. Although evidence gathered from young aggrading forests has generally indicated a strong CO2 fertilization effect on biomass growth3,4,5, it is unclear whether mature forests respond to eCO2 in a similar way. In mature trees and forest stands7,8,9,10, photosynthetic uptake has been found to increase under eCO2 without any apparent accompanying growth response, leaving the fate of additional carbon fixed under eCO2 unclear4,5,7,8,9,10,11. Here using data from the first ecosystem-scale Free-Air CO2 Enrichment (FACE) experiment in a mature forest, we constructed a comprehensive ecosystem carbon budget to track the fate of carbon as the forest responded to four years of eCO2 exposure. We show that, although the eCO2 treatment of +150 parts per million (+38 per cent) above ambient levels induced a 12 per cent (+247 grams of carbon per square metre per year) increase in carbon uptake through gross primary production, this additional carbon uptake did not lead to increased carbon sequestration at the ecosystem level. Instead, the majority of the extra carbon was emitted back into the atmosphere via several respiratory fluxes, with increased soil respiration alone accounting for half of the total uptake surplus. Our results call into question the predominant thinking that the capacity of forests to act as carbon sinks will be generally enhanced under eCO2, and challenge the efficacy of climate mitigation strategies that rely on ubiquitous CO2 fertilization as a driver of increased carbon sinks in global forests.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data included in this paper are available via Figshare (https://doi.org/10.6084/m9.figshare.11634315.v1).
Code availability
Codes for processing the data are available via GitHub (https://github.com/mingkaijiang/EucFACE_carbon_budget_paper.git).
References
Le Quéré, C. L. et al. Global carbon budget 2018. Earth Syst. Sci. Data 10, 2141–2194 (2018).
Schimel, D., Stephens, B. B. & Fisher, J. B. Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl Acad. Sci. USA 112, 436–441 (2015).
Walker, A. P. et al. Decadal biomass increment in early secondary successional woody ecosystems is increased by CO2 enrichment. Nat. Commun. 10, 454 (2019).
Norby, R. J. & Zak, D. R. Ecological lessons from Free-Air CO2 Enrichment (FACE) experiments. Annu. Rev. Ecol. Evol. Syst. 42, 181–203 (2011).
Leuzinger, S. & Hättenschwiler, S. Beyond global change: lessons from 25 years of CO2 research. Oecologia 171, 639–651 (2013).
Arora, V. K. et al. Carbon-concentration and carbon-climate feedbacks in CMIP5 Earth system models. J. Clim. 26, 5289–5314 (2013).
Ellsworth, D. S. et al. Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat. Clim. Chang. 7, 279–282 (2017).
Körner, C. et al. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 309, 1360–1362 (2005).
Ryan, M. G. Three decades of research at Flakaliden advancing whole-tree physiology, forest ecosystem and global change research. Tree Physiol. 33, 1123–1131 (2013).
Klein, T. et al. Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment. J. Ecol. 104, 1720–1733 (2016).
Norby, R. J. et al. Model-data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytol. 209, 17–28 (2016).
Pugh, T. A. M. et al. Role of forest regrowth in global carbon sink dynamics. Proc. Natl Acad. Sci. USA 116, 4382–4387 (2019).
Grassi, G. et al. The key role of forests in meeting climate targets requires science for credible mitigation. Nat. Clim. Chang. 7, 220–226 (2017).
Peñuelas, J. et al. Shifting from a fertilization-dominated to a warming-dominated period. Nat. Ecol. Evol. 1, 1438–1445 (2017).
Luo, Y. et al. Progressive nitrogen limitation of ecosystem response to rising atmospheric carbon dioxide. Bioscience 54, 731–739 (2004).
DeLucia, E. H. et al. Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284, 1177–1179 (1999).
Crous, K., Ósvaldsson, A. & Ellsworth, D. S. Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilization stimulates stem growth. Plant Soil 391, 293–305 (2015).
Medlyn, B. E. et al. Using models to guide field experiments: a priori predictions for the CO2 response of a nutrient- and water-limited native eucalypt woodland. Glob. Change Biol. 22, 2834–2851 (2016).
Medlyn, B. E. et al. Using ecosystem experiments to improve vegetation models. Nat. Clim. Chang. 5, 528–534 (2015).
Friedlingstein, P. et al. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 27, 511–526 (2014).
Yang, J. et al. Low sensitivity of gross primary production to elevated CO2 in a mature eucalypt woodland. Biogeosciences 17, 265–279 (2020).
DeLucia, E. H., Drake, J. E., Thomas, R. B. & Gonzalez-Meler, M. Forest carbon use efficiency: is respiration a constant fraction of gross primary production? Glob. Change Biol. 13, 1157–1167 (2007).
Norby, R. J. Forest canopy productivity index. Nature 381, 564 (1996).
Duursma, R. A. et al. Canopy leaf area of a mature evergreen Eucalyptus woodland does not respond to elevated atmospheric CO2 but tracks water availability. Glob. Change Biol. 22, 1666–1676 (2016).
Drake, J. E. et al. Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Glob. Change Biol. 22, 380–390 (2016).
Drake, J. E. et al. Three years of soil respiration in a mature eucalypt woodland exposed to atmospheric CO2 enrichment. Biogeochemistry 139, 85–101 (2018).
Drake, J. E. et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 (2011).
Hasegawa, S., Macdonald, C. A. & Power, S. A. Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited Eucalyptus woodland. Glob. Change Biol. 22, 1628–1643 (2016).
Ochoa-Hueso, R. et al. Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil 416, 283–295 (2017).
Crous, K. Y., Wujeska-Klause, A., Jiang, M., Medlyn, B. E. & Ellsworth, D. S. Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature Eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 10, 664 (2019).
Zaehle, S. et al. Evaluation of 11 terrestrial carbon-nitrogen cycle models against observations from two temperature Free-Air CO2 Enrichment studies. New Phytol. 202, 803–822 (2014).
Fleischer, K. et al. Amazon forest response to CO2 fertilization dependent on plant phosphorus acquisition. Nat. Geosci. 12, 736–741 (2019).
Todd-Brown, K. E. O. et al. Changes in soil organic carbon storage predicted by earth system models during the 21st century. Biogeosciences 11, 2341–2356 (2014).
Kuzyakov, Y., Horwath, W. R., Dorodnikov, M. & Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: no changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 128, 66–78 (2019).
Luyssaert, S. et al. Old-growth forests as global carbon sinks. Nature 455, 213–215 (2008).
Jones, C. et al. 21st century compatible CO2 emissions and airborne fraction simulated by CMIP5 Earth System models under 4 representative concentration pathways. J. Clim. 26, 4398–4413 (2013).
Australia’s Agriculture, Fisheries And Forestry At A Glance 2012 https://www.agriculture.gov.au/about/publications/glance2012 (Department of Agriculture, Fisheries and Forestry, 2012).
Global Forest Resources Assessment 2000 FAO Forestry Paper 140 (Food and Agricultural Organization of the United Nations, 2001).
Gimeno, T. E., McVicar, T. R., O’Grady, A. P., Tissue, D. T. & Ellsworth, D. S. Elevated CO2 did not affect the hydrological balance of a mature native Eucalyptus woodland. Glob. Change Biol. 24, 3010–3024 (2018).
Hasegawa, S. et al. Elevated CO2 concentrations reduce C4 cover and decrease diversity of understorey plant community in a Eucalyptus woodland. J. Ecol. 106, 1483–1494 (2018).
Pathare, V. S. et al. Water availability affects seasonal CO2-induced photosynthetic enhancement in herbaceous species in a periodically dry woodland. Glob. Change Biol. 23, 5164–5178 (2017).
Paul, K. I. et al. Development and testing of allometric equations for estimating above-ground biomass of mixed-species environmental plantings. For. Ecol. Manage. 310, 483–494 (2013).
Collins, L. et al. Understorey productivity in temperate grassy woodland responds to soil water availability but not to elevated CO2. Glob. Change Biol. 24, 2366–2376 (2018).
Snowdon, P. et al. National Carbon Accounting System Technical Report no. 17 (Australian Greenhouse Office, 2000).
Wallander, H. et al. Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils. Soil Biol. Biochem. 57, 1034–1047 (2013).
Buyer, J. S. & Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 61, 127–130 (2012).
Gherlenda, A. N., Esveld, J. L., Hall, A. A. G., Duursma, R. A. & Riegler, M. Boom and bust: rapid feedback responses between insect outbreak dynamics and canopy leaf area impacted by rainfall and CO2. Glob. Change Biol. 22, 3632–3641 (2016).
Facey, S. L. et al. Atmospheric change causes declines in woodland arthropods and impacts specific trophic groups. Agric. For. Entomol. 19, 101–112 (2017).
Murray, T. J., Tissue, D. T., Ellsworth, D. S. & Riegler, M. Interactive effects of pre-industrial, current and future CO2 and temperature on an insect herbivore of Eucalyptus. Oecologia 171, 1025–1035 (2013).
Trakimas, G. et al. Ecological stoichiometry: a link between developmental speed and physiological stress in an omnivorous insect. Front. Behav. Neurosci. 13, 42 (2019).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Medlyn, B. E. et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Change Biol. 17, 2134–2144 (2011).
Gimeno, T. E. et al. Conserved stomatal behavior under elevated CO2 and varying water availability in a mature woodland. Funct. Ecol. 30, 700–709 (2016).
Yang, J. et al. Incorporating non-stomatal limitation improves the performance of leaf and canopy models at high vapor pressure deficit. Tree Physiol. 39, 1961-1974 (2019).
Martins, C. S. C. et al. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal-temperate forests. Funct. Ecol. 31, 2356–2368 (2017).
Zhang, X. & Wang, W. The decomposition of fine and coarse roots: their global patterns and controlling factors. Sci. Rep. 5, 9940 (2015).
Reich, P. B. et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410, 809–810 (2001).
Gherlenda, A. N., Moore, B. D., Haigh, A. M., Johnson, S. N. & Riegler, M. Insect herbivory in a mature Eucalyptus woodland canopy depends on leaf phenology but not CO2 enrichment. BMC Ecol. 16, 47 (2016).
Gherlenda, A. N. et al. Precipitation, not CO2 enrichment, drives insect herbivore frass deposition and subsequent nutrient dynamics in a mature Eucalyptus woodland. Plant Soil 399, 29–39 (2016).
Drake, J. E. et al. The partitioning of gross primary production for young Eucalyptus tereticornis trees under experimental warming and altered water availability. New Phytol. 222, 1298–1312 (2019).
Salomón, R. L., Steppe, K., Crous, K. Y., Noh, N. J. & Ellsworth, D. S. Elevated CO2 does not affect stem CO2 efflux nor stem respiration in dry Eucalyptus woodland, but it shifts the vertical gradient in xylem CO2. Plant Cell Environ. 42, 2151–2164 (2019).
Raumonen, P. et al. Fast automatic precision tree models from terrestrial laser scanner data. Remote Sens. 5, 491–520 (2013).
Calders, K. et al. Nondestructive estimates of above-ground biomass using terrestrial laser scanning. Methods Ecol. Evol. 6, 198–208 (2015).
Davidson, E. A., Samanta, S., Caramori, S. S. & Savage, K. The dual Arrhenius and Michaelis–Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob. Change Biol. 18, 371–384 (2012).
Guenther, A. B. et al. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 5, 1471–1492 (2012).
Emmerson, K. M., Palmer, P. I., Thatcher, M., Haverd, V. & Guenther, A. B. Sensitivity of isoprene emissions to drought over south-eastern Australia: integrating models and satellite observations of soil moisture. Atmos. Environ. 209, 112–124 (2019).
Kännaste, A., Copolovici, L. & Niinemets, Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In Plant Isoprenoids: Methods And Protocols (ed. Rodríguez-Concepción, M.) 161–169 (Humana Press, 2014).
Tholl, D. et al. Practical approaches to plant volatile analysis. Plant J. 45, 540–560 (2006).
Li, T., Holst, T., Michelsen, A. & Rinnan, R. Amplification of plant volatile defense against insect herbivory in a warming Arctic tundra. Nat. Plants 5, 568–574 (2019).
Johnsen, L. G., Skou, P. B., Khakimov, B. & Bro, R. Gas chromatography—mass spectrometry data processing made easy. J. Chromatogr. A 1503, 57–64 (2017).
Keith, H. et al. Multiple measurements constrain estimates of net carbon exchange by a Eucalyptus forest. Agric. For. Meteorol. 149, 535–558 (2009).
Bates, D., Machler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
R Core Team. R: A Language And Environment For Statistical Computing https://www.R-project.org/ (R Foundation for Statistical Computing, 2018).
Ouimette, A. P. et al. Accounting for carbon flux to mycorrhizal fungi may resolve discrepancies in forest carbon budgets. Ecosystems https://doi.org/10.1007/s10021-019-00440-3 (2019).
Harris, I., Jones, P. D., Osborn, T. J. & Lister, D. H. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 dataset. Int. J. Climatol. 34, 623–642 (2014).
Olson, D. M. et al. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51, 933–938 (2001).
Jiang, M., Felzer, B. S., Nielsen, U. N. & Medlyn, B. E. Biome-specific climatic space defined by temperature and precipitation predictability. Glob. Ecol. Biogeogr. 26, 1270–1282 (2017).
Scarascia-Mugnozza, G. et al. Response to elevated CO2 of a short rotation, multispecies Poplar plantation: the POPFACE/EUROFACE experiment. In Managed Ecosystems and CO 2 (eds. Nösberger, J. et al.) 173–195 (Springer, 2006).
Linder, S. NPP Boreal Forest: Flakaliden, Sweden, 1986-1996, R1. Dataset at https://doi.org/10.3334/ORNLDAAC/201 (Oak Ridge National Laboratory Distributed Active Archive Center, 2013).
Anderson-Teixeira, K. J. et al. ForC: a global database of forest carbon stock and fluxes. Ecology 99, 1507 (2018).
Shangguan, W., Dai, Y., Duan, Q., Liu, B. & Yuan, H. A global soil data set for Earth system modelling. J. Adv. Model. Earth Syst. 6, 249–263 (2014).
Yang, X., Post, W.M., Thornton, P.E. and Jain, A. Global gridded soil phosphorus distribution maps at 0.5-degree resolution. Dataset at https://doi.org/10.3334/ORNLDAAC/1223 (Oak Ridge National Laboratory Distributed Active Archive Center, 2014).
Acknowledgements
EucFACE was built as an initiative of the Australian Government as part of the Nation-building Economic Stimulus Package, and is supported by the Australian Commonwealth in collaboration with Western Sydney University. We acknowledge technical support by V. Kumar, C. McNamara and S. Wohl, and the team who assisted with data collection. This work was partially supported by the following grants from the Australian Research Council (ARC): DP130102501 (to J.R.P. and I.C.A.), DP170104634 (to B.K.S. and P.B.R.), DP170102766 (to E.P. and M.G.T.), DP110105102 and DP160102452 (to D.S.E.). M.G.D.K. acknowledges funding from the ARC Centre of Excellence for Climate Extremes (CE170100023), the ARC Discovery Grant (DP190101823) and support from the NSW Research Attraction and Acceleration Program. R.L.S received funding from Research Foundation Flanders and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 665501. R.O.-H. is financially supported by a Ramón y Cajal Fellowship from MICIU (RYC-2017-22032). E.H.J.N. and B.M.d.S. received funding from the VILLUM Center for Plant Plasticity (VKR023054), the VILLUM Young Investor Program fellowship (VKR013167), and a Danish Independent Research Council Sapere Aude Research Talent Post-Doctoral Stipend (6111-00379B). Ü.N. and A.K. were supported by the European Commission through the European Regional Fund (Center of Excellence EcolChange). S.Z. was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 647204, QUINCY).
Author information
Authors and Affiliations
Contributions
M.J., B.E.M., R.A.D. and J.E.D. designed the synthesis, compiled the data and performed the analyses. M.J., B.E.M., J.E.D., R.A.D., I.C.A., C.V.M.B., M.M.B., Y.C., L.C.-G., L.C., K.Y.C., B.M.d.S., S.L.F., A.N.G., T.E.G., S.H., S.N.J., A.K., C.A.M., K.M., B.D.M., L.N., E.H.J.N., U.N.N., Ü.N., N.J.N., R.O.-H., V.S.P., E.P., J. Pihlblad, J. Pineiro, J.R.P., S.A.P., P.B.R., A.A.R., M.R., R.R., P.D.R., R.L.S., B.K.S., B.S., M.G.T., J.K.M.W., A.W.-K., J.Y. and D.S.E. collected data and contributed to data analyses. M.J. performed data assimilation analysis, with contributions from M.G.D.K. and B.E.M. J.Y. and B.E.M. performed the MAESPA model simulations, with contributions from M.G.D.K. and R.A.D. J.E.D. and A.A.R. performed soil respiration gap-filling and modelling. K.M.E. performed the MEGAN model simulation. M.J. and L.C.-G. conceptualized Fig. 1, and L.C.-G. implemented the graphic design and hand-drew all the vectors in the figure. M.J. wrote the initial manuscript, with substantial input from B.E.M., J.E.D., B.S., P.B.R., S.Z., M.G.D.K., M.G.T. and D.S.E. All authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Kirsti Ashworth, Benjamin Bond-Lamberty, Yiqi Luo and Andrew Richardson for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The EucFACE experiment facility.
a, View of the forest and facility from above (photograph taken by D.S.E.). b, View of the understorey vegetation and infrastructure inside a plot (photograph taken by M.J.). c, View from below of the canopy structure and the crane (photograph taken by M.J.).
Extended Data Fig. 2 Mean annual temperature (MAT) and mean annual precipitation (MAP) for major forest biomes and a selected list of tree-based elevated CO2 experiments.
Gridded temperature and precipitation data were obtained from the Climate Research Unit monthly dataset at 0.5-degree resolution75. Global biome boundaries and definitions were taken from ref. 76 and were spatially aggregated onto the Climate Research Unit resolution, following ref. 77. Only forest biomes were included here, including: tropical and subtropical moist broadleaf forests; tropical and subtropical dry broadleaf forests; tropical and subtropical coniferous forest; temperate broadleaf and mixed forests; temperate coniferous forests; boreal forests/taiga; and Mediterranean forests, woodlands, and scrub. The list of elevated CO2 experiments includes seven FACE experiments and a Whole-Tree Chamber (WTC) experiment, namely: EucFACE, DukeFACE, ORNLFACE, AspenFACE, PopFACE, WebFACE, BiForFACE and FlakalidenWTC. The site-specific climate, tree age and net primary production (NPP) under ambient CO2 treatment were collected from refs. 3,9,10,11,78,79. The top inset compares global forest NPP against standing age using data collected from ref. 80. We included data with forest age <500 years, and the NPP reported in ref. 80 includes both overstorey and understorey. The bottom inset compares soil total nitrogen and labile phosphorus across the eCO2 experiments. Soil total nitrogen was extracted from ref. 81 using the spatial coordinates of each experiment, while soil labile phosphorus was spatially extracted from ref. 82. The two dotted lines indicate N:P ratios of 20:1 and 100:1, respectively. The count in the colour scale refers to the number of grids that fall within each climate space.
Extended Data Fig. 3 Estimates of GPP (a, b) and soil respiration (Rsoil) (c, d) based on different methods for both ambient (a, c) and elevated (b, d) CO2 treatment at EucFACE.
For estimates of GPP, we compared the model simulated total GPP of overstorey and understorey (GPPo and GPPu, respectively), with the sum of data-driven estimates of net primary production (NPP) and autotrophic respiration (Ra), which include NPP of overstorey leaf (NPPol), stem (NPPstem), fine root (NPPfroot), intermediate root (NPPiroot), coarse root (NPPcroot), twigs, barks and seeds (NPPother), understorey aboveground (NPPua), leaf consumption by insects (NPPins), and respiratory fluxes of overstorey leaf (Rol), stem (Rstem), root (Rroot), understorey aboveground (Rua), growth (Rgrow), and volatile carbon emission (VC). For estimates of Rsoil, we compared direct estimates of Rsoil scaled up from soil chamber measurements, with the sum of litterfall and independent estimates of root respiration (Litter + Rroot), assuming no net change in soil carbon stock over time. Here litterfall was inferred from the NPP of overstorey leaf (NPPol), fine root (NPPfroot), intermediate root (NPPiroot), twigs, barks and seeds (NPPother), understorey aboveground (NPPua), and frass production (Frass). These evaluations provide independent mass balance checks of the estimated ecosystem carbon budget. Each colour represents a flux variable. Each point and vertical line represent treatment mean and standard deviation, respectively, based on plot-level estimates of the aggregated flux (n = 3). Values were normalized by a linear mixed model with pre-treatment LAI as a covariate to account for pre-existing differences.
Extended Data Fig. 4 The fate of additional carbon fixed under elevated CO2 (eCO2) in a mature forest ecosystem (non-normalized analysis case).
a, GPP represents the total eCO2 induced increase in GPPo and GPPu, respectively, NPP + Ra represents the sum of net primary production and autotrophic respiration eCO2 response, and R + ΔCpool represents the sum of ecosystem respiration and carbon storage eCO2 response. b, The relative contributions of individual NPP fluxes to the aggregated NPP response to eCO2, including overstorey leaf (NPPol), stem (NPPstem), fine root (NPPfroot) and understorey aboveground (NPPua). c, The relative contributions of individual respiratory fluxes to the aggregated R response to eCO2, including overstorey leaf (Rol), stem (Rstem), root (Rroot), understorey aboveground (Rua), and heterotroph (Rhetero). d, The relative contributions of individual change in carbon storage to the aggregated ΔCpool response to eCO2, including stem (ΔCstem), fine root (ΔCfroot), leaflitter (ΔClit), understorey aboveground (ΔCua), and soil (ΔCsoil). Variables with an average CO2 effect of <5 g C m−2 yr−1 were excluded from the figure for better visual clarification. Each colour represents a flux variable, each point indicates the net sum of all variables for a column, and the grey error bar represents the plot-level standard deviation (n = 3) of the estimated column sum.
Extended Data Fig. 5 CO2 treatment effect for all ecosystem fluxes at EucFACE.
a, The CO2 response of gross ecosystem carbon uptake, including GPPo and GPPu, and soil methane uptake (CH4). b, The eCO2 response of annual incremental change in carbon pool (ΔCpool), including overstorey leaf (ΔCol), stem (ΔCstem), coarse root (ΔCcroot), fine root (ΔCfroot), intermediate root (ΔCiroot), understorey aboveground (ΔCua), leaf litter (ΔClit), soil (ΔCsoil), microbe (ΔCmicr), aboveground insect (ΔCins), and mycorrhizae (ΔCmyco). c, The eCO2 response of NPP, including overstorey leaf (NPPol), stem (NPPstem), coarse root (NPPcroot), fine root (NPPfroot), intermediate root (NPPiroot), understorey aboveground (NPPua), twigs, barks and seeds (NPPother), and leaf insect consumption (NPPins). d, The eCO2 response of ecosystem R and other outgoing fluxes, including respiration fluxes of overstorey leaf (Rol), stem (Rstem), root (Rroot), understorey aboveground (Rua), growth (Rgrow), insect (Rins), heterotroph (Rhetero), and soil (Rsoil), and volatile carbon emission (VC) and DOC leaching. Dots and grey bars represent means and standard deviations of the CO2 treatment difference, predicted by a linear mixed model with plot-specific pre-treatment LAI as a covariate. Red dots indicate negative means and blue dots indicate positive means. Dashed lines indicate change of scale along the x-axis.
Extended Data Fig. 6 Estimates of incremental change in carbon pool averaged over the experimental period under ambient (aCO2) and elevated CO2 (eCO2) treatment effect at EucFACE (ΔCpool).
The ΔCpool variables are overstorey leaf (ΔCol), stem (ΔCstem), coarse root (ΔCcroot), fine root (ΔCfroot), intermediate root (ΔCiroot), understorey aboveground (ΔCua), leaf litter (ΔClit), soil (ΔCsoil), microbe (ΔCmicr), aboveground insect (ΔCins), and mycorrhizae (ΔCmyco). Each coloured bar and black line (error bar) represents the mean and standard deviation for each treatment, with blue representing aCO2 and red representing eCO2 treatment. Dashed lines indicate change of scale along the x axis.
Extended Data Fig. 7 Fitted carbon cycle parameters to trace the fate of the additional carbon under elevated CO2 at EucFACE.
Parameters were estimated by MCMC fitting algorithm, assuming a simplified carbon cycle framework based on data collected from EucFACE. Details of the MCMC approach can be found in the Methods. Plot-level GPP, autotrophic respiration (Ra), heterotrophic respiration (Rhetero), carbon pools of leaf (C′leaf), wood (C′wood), root (C′root), mycorrhizae (C′myco), microbe (C′micr), and soil (C′soil), and the corresponding change in pools were used to constrain the model fitting. NPP was derived as the difference of GPP and Ra. Carbon use efficiency (CUE′) was calculated as NPP/GPP; it differs from the value given in the main text owing to the contribution of NPP allocated to mycorrhizae (NPPmyco). We fitted two carbon pools (C′aglit and C′bglit), four allocation coefficients (aleaf, awood, aroot and amyco), six turnover rates (τleaf, τroot, τmyco, τbglit, τmicr and τsoil), and three fractional coefficients (f ′aglit, f ′bglit and f ′micr) using the MCMC algorithm. The fractional coefficients indicate the fraction of carbon leaving one pool that enters the subsequent pool, with the remainder respired as Rhetero.
Extended Data Fig. 8 Data–model intercomparison of some key carbon cycle parameters, under ambient (aCO2) and elevated CO2 (eCO2).
Parameters include: a, allocation coefficients to leaf, wood, root and other; b, turnover rates of leaf, root, aboveground litter (Aglit), belowground litter (Bglit); and c, turnover rate of soil. Models include: Community Atmosphere Biosphere Land Exchange (CABL), Community Land Model 4 (CLM4), Community Land Model with a phosphorus component (CLMP), Generic Decomposition And Yield (GDAY), Lund-Potsdam-Jena General Ecosystem Simulator (LPJX), Orchidee-C-N (OCNX) and Sheffield Dynamic Global Vegetation Model (SDVM). The model output was generated as part of the model ensemble predictions made in advance of the experiment reported in ref. 17 for EucFACE. Database uncertainties were estimated using the MCMC data assimilation algorithm, with error bars indicating one standard deviation. Allocation to ‘Other’ in the data refers to the allocation to mycorrhizal production, whereas in some models it refers to the allocation to reproductive carbon pool.
Supplementary information
Supplementary Information
This file contains Supplementary Tables S1–S2 and Supplementary Figures S1–S20.
Rights and permissions
About this article
Cite this article
Jiang, M., Medlyn, B.E., Drake, J.E. et al. The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580, 227–231 (2020). https://doi.org/10.1038/s41586-020-2128-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2128-9
This article is cited by
-
Elevated CO2 levels promote both carbon and nitrogen cycling in global forests
Nature Climate Change (2024)
-
Transition from positive to negative indirect CO2 effects on the vegetation carbon uptake
Nature Communications (2024)
-
Erosion-induced recovery CO2 sink offset the horizontal soil organic carbon removal at the basin scale
Science China Earth Sciences (2024)
-
Aligning climate scenarios to emissions inventories shifts global benchmarks
Nature (2023)
-
Grassland responses to elevated CO2 determined by plant–microbe competition for phosphorus
Nature Geoscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.