Abstract
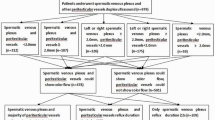
A subset of men with prostate cancer have elevated periprostatic androgens compared with levels in peripheral blood (termed the sneaky T phenomenon), which are associated with poor clinical outcomes after radical prostatectomy. These androgens are of testicular origin and reach the prostate, presumably through venous shunting. Varicocele physiology is accompanied by increased hydrostatic pressure within the pelvic venous system, providing a theoretical mechanistic explanation for the sneaky T phenomenon. These observations suggest a potential role for varicocele in contributing to prostate cancer pathophysiology through sneaky T, which if proved, could be a further indication for varicocele repair. Sneaky T can help to explain the differences in the natural history of benign or malignant prostatic diseases between individuals and could be a tool when deciding on the therapeutic course to take.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wilson, J. D., George, F. W. & Griffin, J. E. The hormonal control of sexual development. Science 211, 1278–1284 (1981).
Deslypere, J. P., Young, M., Wilson, J. D. & McPhaul, M. J. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol. Cell Endocrinol. 88, 15–22 (1992).
Dai, C., Dehm, S. M. & Sharifi, N. Targeting the androgen signaling axis in prostate cancer. J. Clin. Oncol. 41, 4267–4278 (2023).
Auchus, R. J. & Sharifi, N. Sex hormones and prostate cancer. Annu. Rev. Med. 71, 33–45 (2020).
Beveridge, T. S. et al. Retroperitoneal lymph node dissection: anatomical and technical considerations from a cadaveric study. J. Urol. 196, 1764–1771 (2016).
Ku, S. Y., Gleave, M. E. & Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 16, 645–654 (2019).
Winters, S. J. & Troen, P. Testosterone and estradiol are co-secreted episodically by the human testis. J. Clin. Invest. 78, 870–873 (1986).
Alyamani, M. et al. Elevated periprostatic venous testosterone correlates with prostate cancer progression after radical prostatectomy. J. Clin. Invest. 133, e171117 (2023).
Walsh, T. J. et al. Increased risk of high‐grade prostate cancer among infertile men. Cancer 116, 2140–2147 (2010).
Hamada, A., Esteves, S. C. & Agarwal, A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat. Rev. Urol. 10, 26–37 (2013).
Alsaikhan, B., Alrabeeah, K., Delouya, G. & Zini, A. Epidemiology of varicocele. Asian J. Androl. 18, 179–181 (2016).
Liguori, G. et al. Color doppler ultrasound investigation of varicocele. World J. Urol. 22, 378–381 (2004).
Jensen, C. F. S. et al. Varicocele and male infertility. Nat. Rev. Urol. 14, 523–533 (2017).
Sakamoto, H. & Ogawa, Y. Is varicocele associated with underlying venous abnormalities? Varicocele and the prostatic venous plexus. J. Urol. 180, 1427–1431 (2008).
Tilki, D. et al. The complex structure of the smooth muscle layer of spermatic veins and its potential role in the development of varicocele testis. Eur. Urol. 51, 1402–1409 (2007).
Gat, Y., Joshua, S., Vuk-Pavlovic, S. & Goren, M. Paying the price for standing tall: fluid mechanics of prostate pathology. Prostate 80, 1297–1303 (2020).
Black, C. M. Anatomy and physiology of the lower-extremity deep and superficial veins. Tech. Vasc. Interv. Radiol. 17, 68–73 (2014).
Gat, Y., Joshua, S. & Gornish, M. G. Prostate cancer: a newly discovered route for testosterone to reach the prostate : treatment by super-selective intraprostatic androgen deprivation. Andrologia 41, 305–315 (2009).
Hoffman, M. A., DeWOLF, W. C. & Morgentaler, A. Is low serum free testosterone a marker for high grade prostate cancer? J. Urol. 163, 824–827 (2000).
Morgentaler, A., Bruning, C. O. 3rd & DeWolf, W. C. Occult prostate cancer in men with low serum testosterone levels. JAMA 276, 1904–1906 (1996).
Goren, M. & Gat, Y. Varicocele is the root cause of BPH: destruction of the valves in the spermatic veins produces elevated pressure which diverts undiluted testosterone directly from the testes to the prostate. Andrologia 50, e12992 (2018).
Canales, B. K. et al. Prevalence and effect of varicoceles in an elderly population. Urology 66, 627–631 (2005).
Besiroglu, H., Otunctemur, A., Dursun, M. & Ozbek, E. The prevalence and severity of varicocele in adult population over the age of forty years old: a cross-sectional study. Aging Male 22, 207–213 (2019).
Levinger, U., Gornish, M., Gat, Y. & Bachar, G. N. Is varicocele prevalence increasing with age? Andrologia 39, 77–80 (2007).
Wishahi, M. M. Detailed anatomy of the internal spermatic vein and the ovarian vein. Human cadaver study and operative spermatic venography: clinical aspects. J. Urol. 145, 780–784 (1991).
Sofikitis, N., Dritsas, K., Miyagawa, I. & Koutselinis, A. Anatomical characteristics of the left testicular venous system in man. Arch. Androl. 30, 79–85 (1993).
Iafrate, M. et al. Varicocele is associated with an increase of connective tissue of the pampiniform plexus vein wall. World J. Urol. 27, 363–369 (2009).
Li, R., Liu, J., Li, Y. & Wang, Q. Effect of somatometric parameters on the prevalence and severity of varicocele: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 19, 11 (2021).
Zuccolo, L. et al. Height and prostate cancer risk: a large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol. Biomark. Prev. 17, 2325–2336 (2008).
Lophatananon, A. et al. Height, selected genetic markers and prostate cancer risk: results from the PRACTICAL consortium. Br. J. Cancer 117, 734–743 (2017).
Emerging Risk Factors Collaboration. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. Int. J. Epidemiol. 41, 1419–1433 (2012).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. Cancer res. 1, 293–297 (1941).
Tanrikut, C. et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 108, 1480–1484 (2011).
Chang, K. H. & Sharifi, N. Prostate cancer-from steroid transformations to clinical translation. Nat. Rev. Urol. 9, 721–724 (2012).
Ando, S. et al. Physiopathologic aspects of Leydig cell function in varicocele patients. J. Androl. 5, 163–170 (1984).
Zheng, Y. Q. et al. The effects of artery-ligating and artery-preserving varicocelectomy on the ipsilateral testes in rats. Urology 72, 1179–1184 (2008).
Gat, Y., Gornish, M., Heiblum, M. & Joshua, S. Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment. Andrologia 40, 273–281 (2008).
Jarow, J. P., Chen, H., Rosner, T. W., Trentacoste, S. & Zirkin, B. R. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J. Androl. 22, 640–645 (2001).
Jarow, J. P., Wright, W. W., Brown, T. R., Yan, X. & Zirkin, B. R. Bioactivity of androgens within the testes and serum of normal men. J. Androl. 26, 343–348 (2005).
Han, H. et al. Significant alterations of serum hormone levels in the spermatic vein plexus of patients with varicoceles. Andrologia 48, 1108–1112 (2016).
Lima, T. F. N., Patel, P., Blachman-Braun, R., Madhusoodanan, V. & Ramasamy, R. Serum 17-hydroxyprogesterone is a potential biomarker for evaluating intratesticular testosterone. J. Urol. 204, 551–556 (2020).
Engels, M. et al. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr. Rev. 40, 973–987 (2019).
Luo, D. Y., Yang, G., Liu, J. J., Yang, Y. R. & Dong, Q. Effects of varicocele on testosterone, apoptosis and expression of StAR mRNA in rat Leydig cells. Asian J. Androl. 13, 287–291 (2011).
Bernstein, A. P. & Najari, B. B. Varicocele treatment and serum testosterone. Androgens: Clin. Res. Ther. 3, 133–137 (2022).
Tu, H. et al. Low serum testosterone is associated with tumor aggressiveness and poor prognosis in prostate cancer. Oncol. Lett. 13, 1949–1957 (2017).
Welén, K., Damber, J.-E. J. R. I. E. & Disorders, M. Androgens, aging, and prostate health. Rev. Endocr. Metab. Disord. 23, 1221–1231 (2022).
Roehrborn, C. G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 20, S11–S18 (2008).
Chughtai, B. et al. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2, 16032 (2016).
Berry, S. J., Coffey, D. S., Walsh, P. C. & Ewing, L. L. The development of human benign prostatic hyperplasia with age. J. Urol. 132, 474–479 (1984).
Page, S. T. et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J. Clin. Endocrinol. Metab. 96, 430–437 (2011).
van der Sluis, T. M. et al. Intraprostatic testosterone and dihydrotestosterone. Part II: concentrations after androgen hormonal manipulation in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 109, 183–188 (2012).
Dai, C. et al. Direct metabolic interrogation of dihydrotestosterone biosynthesis from adrenal precursors in primary prostatectomy tissues. Clin. Cancer Res. 23, 6351–6362 (2017).
Carson, C. III & Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 61, 2–7 (2003).
Kim, I. Y. et al. Modulation of sensitivity to transforming growth factor-beta 1 (TGF-beta 1) and the level of type II TGF-beta receptor in LNCaP cells by dihydrotestosterone. Exp. Cell Res. 222, 103–110 (1996).
Foster, C. S. Pathology of benign prostatic hyperplasia. Prostate Suppl. 9, 4–14 (2000).
Griffiths, K., Morton, M. S. & Nicholson, R. I. Androgens, androgen receptors, antiandrogens and the treatment of prostate cancer. Eur. Urol. 32, 24–40 (1997).
Isaacs, J. T. Antagonistic effect of androgen on prostatic cell death. Prostate 5, 545–557 (1984).
Niu, Y. et al. Proliferation and differentiation of prostatic stromal cells. BJU Int. 87, 386–393 (2001).
Kim, E. H., Brockman, J. A. & Andriole, G. L. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J. Urol. 5, 28–32 (2018).
Thirumalai, A. et al. Stable intraprostatic dihydrotestosterone in healthy medically castrate men treated with exogenous testosterone. J. Clin. Endocrinol. Metab. 101, 2937–2944 (2016).
Parsons, J. K., Palazzi-Churas, K., Bergstrom, J. & Barrett-Connor, E. Prospective study of serum dihydrotestosterone and subsequent risk of benign prostatic hyperplasia in community dwelling men: the Rancho Bernardo Study. J. Urol. 184, 1040–1044 (2010).
Trifiro, M. D. et al. Serum sex hormones and the 20-year risk of lower urinary tract symptoms in community-dwelling older men. BJU Int. 105, 1554–1559 (2010).
Kristal, A. R. et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am. J. Epidemiol. 168, 1416–1424 (2008).
Schatzl, G. et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate 47, 52–58 (2001).
Kratzik, C. et al. Lower serum total testosterone is associated with lymph node metastases in a radical prostatectomy cohort study. Anticancer Res. 31, 3615–3618 (2011).
Li, X. et al. BMX controls 3betaHSD1 and sex steroid biosynthesis in cancer. J. Clin. Invest. 133, e163498 (2023).
Waltering, K. K. et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 69, 8141–8149 (2009).
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Nishiyama, T., Hashimoto, Y. & Takahashi, K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin. Cancer Res. 10, 7121–7126 (2004).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. M.B., R.R. and N.S. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Helen Bernie, Karl Storbeck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bitaraf, M., Ramasamy, R., Punnen, S. et al. Elevated periprostatic androgens, sneaky testosterone and its implications. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00878-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00878-8