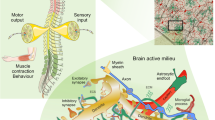
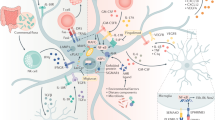
Abstract
Epilepsy affects ~65 million people worldwide. First-line treatment options include >20 antiseizure medications, but seizure control is not achieved in approximately one-third of patients. Antiseizure medications act primarily on neurons and can provide symptomatic control of seizures, but do not alter the onset and progression of epilepsy and can cause serious adverse effects. Therefore, medications with new cellular and molecular targets and mechanisms of action are needed. Accumulating evidence indicates that astrocytes are crucial to the pathophysiological mechanisms of epilepsy, raising the possibility that these cells could be novel therapeutic targets. In this Review, we discuss how dysregulation of key astrocyte functions — gliotransmission, cell metabolism and immune function — contribute to the development and progression of hyperexcitability in epilepsy. We consider strategies to mitigate astrocyte dysfunction in each of these areas, and provide an overview of how astrocyte activation states can be monitored in vivo not only to assess their contribution to disease but also to identify markers of disease processes and treatment effects. Improved understanding of the roles of astrocytes in epilepsy has the potential to lead to novel therapies to prevent the initiation and progression of epilepsy.
Key points
-
In health, astrocytes have key functions in the CNS that are compromised in disease states such as epilepsy; this astrocytic dysfunction contributes to pathological changes in synaptic transmission and to hyperexcitability.
-
Adenosine dysfunction in epilepsy is tightly linked to astrocyte pathophysiology.
-
Astrocyte-mediated inflammation can promote epileptogenesis and seizure recurrence, especially when endogenous anti-inflammatory molecules, such as IL-1 receptor antagonist, IL-10 and proresolving lipid mediators, fail to resolve inflammation.
-
Imaging of astrocyte activation states and fluid biomarkers could form the basis of prognostic and diagnostic markers of epilepsy that facilitate patient stratification in clinical studies.
-
Resolution of astrocytic dysfunction that contributes to pathogenesis in epilepsy is a new frontier for therapeutic development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Khakh, B. S. & Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019). This review emphasizes that astrocytes are diverse and have properties and functions specific to particular brain areas and diseases; gaps in our understanding of astrocyte diversity and its physiological relevance in the CNS are identified.
Archie, S. R., Al Shoyaib, A. & Cucullo, L. Blood-brain barrier dysfunction in CNS disorders and putative therapeutic targets: an overview. Pharmaceutics 13, 1779 (2021).
MacVicar, B. A. & Newman, E. A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 7, a020388 (2015).
Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215 (1999).
Devinsky, O., Vezzani, A., Najjar, S., De Lanerolle, N. C. & Rogawski, M. A. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184 (2013).
Verhoog, Q. P., Holtman, L., Aronica, E. & van Vliet, E. A. Astrocytes as guardians of neuronal excitability: mechanisms underlying epileptogenesis. Front. Neurol. 11, 591690 (2020).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Middeldorp, J. & Hol, E. M. GFAP in health and disease. Prog. Neurobiol. 93, 421–443 (2011).
Ammothumkandy, A. et al. Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nat. Neurosci. 25, 493–503 (2022). This study shows that a longer duration of epilepsy is associated with a sharp decline in neurogenesis but maintained astrogenesis, and that immature neurons in mesial TLE are mostly inactive but the activity of immature astrocytes in the hippocampus depends on epileptiform-like activity.
Yang, L. et al. Astrocyte activation and memory impairment in the repetitive febrile seizures model. Epilepsy Res. 86, 209–220 (2009).
Devinsky, O. et al. Epilepsy. Nat. Rev. Dis. Prim. 4, 18024 (2018).
Bedner, P., Jabs, R. & Steinhäuser, C. Properties of human astrocytes and NG2 glia. Glia 68, 756–767 (2020).
Li, J. et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 12, 3958 (2021).
Eid, T., Tu, N., Lee, T.-S. W. & Lai, J. C. K. Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem. Int. 63, 670–681 (2013).
Bedner, P. et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138, 1208–1222 (2015). This study provides the first direct evidence that loss of astrocytic gap junction coupling has a key role in the initiation and progression of epilepsy.
Messing, A., Brenner, M., Feany, M. B., Nedergaard, M. & Goldman, J. E. Alexander disease. J. Neurosci. 32, 5017–5023 (2012).
Feige, L., Zaeck, L. M., Sehl-Ewert, J., Finke, S. & Bourhy, H. Innate immune signaling and role of glial cells in herpes simplex virus- and rabies virus-induced encephalitis. Viruses 13, 2364 (2021).
Robel, S. et al. Reactive astrogliosis causes the development of spontaneous seizures. J. Neurosci. 35, 3330–3345 (2015).
Ortinski, P. I. et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat. Neurosci. 13, 584–591 (2010).
Shao, L. et al. Silencing of circIgf1r plays a protective role in neuronal injury via regulating astrocyte polarization during epilepsy. FASEB J. 35, e21330 (2021).
Sano, F. et al. Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus. JCI Insight 6, 135391 (2021).
Maupu, C. et al. Diisopropylfluorophosphate-induced status epilepticus drives complex glial cell phenotypes in adult male mice. Neurobiol. Dis. 152, 105276 (2021).
Aronica, E., Ravizza, T., Zurolo, E. & Vezzani, A. Astrocyte immune response in epilepsy. Glia 60, 1258–1268 (2012).
Vezzani, A., Balosso, S. & Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 15, 459–472 (2019).
Klein, P. et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia 59, 37–66 (2018).
Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192 (2002).
Savtchouk, I. & Volterra, A. Gliotransmission: beyond black-and-white. J. Neurosci. 38, 14–25 (2018).
Fiacco, T. A. & McCarthy, K. D. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci. 38, 3–13 (2018).
Parpura, V. et al. Glutamate-mediated astrocyte–neuron signalling. Nature 369, 744–747 (1994).
Nedergaard, M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771 (1994).
Bezzi, P. et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613 (2004). This study provides the first proof that transmitter vesicles are present in astrocytes in situ.
Bohmbach, K., Schwarz, M. K., Schoch, S. & Henneberger, C. The structural and functional evidence for vesicular release from astrocytes in situ. Brain Res. Bull. 136, 65–75 (2018).
Fellin, T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743 (2004).
Angulo, M. C., Kozlov, A. S., Charpak, S. & Audinat, E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927 (2004).
Jourdain, P. et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339 (2007).
Araque, A., Castillo, P. E., Manzoni, O. J. & Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24 (2017).
Henneberger, C., Papouin, T., Oliet, S. H. R. & Rusakov, D. A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010).
Papouin, T., Dunphy, J., Tolman, M., Foley, J. C. & Haydon, P. G. Astrocytic control of synaptic function. Philos. Tran R. Soc. Lond. B Biol. Sci. 372, 20160154 (2017).
Araque, A., Martín, E. D., Perea, G., Arellano, J. I. & Buño, W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J. Neurosci. 22, 2443–2450 (2002).
Beamer, E., Kuchukulla, M., Boison, D. & Engel, T. ATP and adenosine – two players in the control of seizures and epilepsy development. Prog. Neurobiol. 204, 102105 (2021).
Yoon, B.-E. & Lee, C. J. GABA as a rising gliotransmitter. Front. Neural Circuits 8, 141 (2014).
Cavus, I. et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 57, 226–235 (2005).
Soukupova, M. et al. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscience 301, 246–253 (2015).
Tian, G.-F. et al. An astrocytic basis of epilepsy. Nat. Med. 11, 973–981 (2005).
Kang, N., Xu, J., Xu, Q., Nedergaard, M. & Kang, J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130 (2005).
Fellin, T., Gomez-Gonzalo, M., Gobbo, S., Carmignoto, G. & Haydon, P. G. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J. Neurosci. 26, 9312–9322 (2006).
Gómez-Gonzalo, M. et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 8, e1000352 (2010).
Heuser, K. et al. Ca2+ signals in astrocytes facilitate spread of epileptiform activity. Cereb. Cortex 28, 4036–4048 (2018).
Clasadonte, J., Dong, J., Hines, D. J. & Haydon, P. G. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proc. Natl Acad. Sci. USA 110, 17540–17545 (2013). This study shows that genetic inhibition of the astrocytic transmitter release machinery attenuates epileptiform activity and neurodegeneration through a pathway involving NMDA receptors.
Riquelme, J., Wellmann, M., Sotomayor-Zárate, R. & Bonansco, C. Gliotransmission: a novel target for the development of antiseizure drugs. Neuroscientist 26, 293–309 (2020).
Seifert, G., Hüttmann, K., Schramm, J. & Steinhäuser, C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon’s horn sclerosis. J. Neurosci. 24, 1996–2003 (2004).
Seifert, G., Schilling, K. & Steinhauser, C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206 (2006).
Beamer, E., Conte, G. & Engel, T. ATP release during seizures – a critical evaluation of the evidence. Brain Res. Bull. 151, 65–73 (2019).
Dossi, E. et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 10, eaar3796 (2018).
Jimenez-Pacheco, A. et al. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 54, 1551–1561 (2013).
Alves, M. et al. Context-specific switch from anti- to pro-epileptogenic function of the P2Y1 receptor in experimental epilepsy. J. Neurosci. 39, 5377–5392 (2019).
Alves, M. et al. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 58, 1603–1614 (2017).
Álvarez-Ferradas, C. et al. Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 63, 1507–1521 (2015).
Nikolic, L. et al. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 66, 2673–2683 (2018). This study reveals that pathologically elevated tumour necrosis factor contributes to epileptogenesis by boosting hyperexcitation through an autocrine mechanism that involves purinergic signalling, astrocyte glutamate release and modulation of neuronal transmitter release.
Wellmann, M., Álvarez-Ferradas, C., Maturana, C. J., Sáez, J. C. & Bonansco, C. Astroglial Ca2+-dependent hyperexcitability requires P2Y1 purinergic receptors and pannexin-1 channel activation in a chronic model of epilepsy. Front. Cell Neurosci. 12, 446 (2018).
Alves, M., Smith, J. & Engel, T. Differential expression of the metabotropic P2Y receptor family in the cortex following status epilepticus and neuroprotection via P2Y1 antagonism in mice. Front. Pharmacol. 10, 558 (2020).
Pavlov, I. & Walker, M. C. Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology 69, 55–61 (2013).
Müller, J. et al. Astrocytic GABA accumulation in experimental temporal lobe epilepsy. Front. Neurol. 11, 614923 (2020).
Pandit, S. et al. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 68, 1065–1080 (2020). This study shows that reactive astrocytes in experimental TLE aberrantly overproduce and release GABA, which in turn inhibits neuronal excitability and network activity through activation of tonic GABAA receptor-mediated currents in excitatory neurons.
Aronica, E. et al. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur. J. Neurosci. 12, 2333–2344 (2000).
Aronica, E. et al. Ionotropic and metabotropic glutamate receptor protein expression in glioneuronal tumours from patients with intractable epilepsy. Neuropathol. Appl. Neurobiol. 27, 223–237 (2001).
Jimenez-Pacheco, A. et al. Transient P2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J. Neurosci. 36, 5920–5932 (2016).
Fischer, W. et al. Critical evaluation of P2X7 receptor antagonists in selected seizure models. PLoS ONE 11, e0156468 (2016).
Natelson, S., Miletich, D. J., Seals, C. F., Visintine, D. J. & Albrecht, R. F. Clinical biochemistry of epilepsy. I. Nature of the disease and a review of the chemical findings in epilepsy. Clin. Chem. 25, 889–897 (1979).
Boison, D. The biochemistry and epigenetics of epilepsy: focus on adenosine and glycine. Front. Mol. Neurosci. 9, 26 (2016).
Patel, M. A metabolic paradigm for epilepsy. Epilepsy Curr. 18, 318–322 (2018).
Boison, D. & Steinhäuser, C. Epilepsy and astrocyte energy metabolism. Glia 66, 1235–1243 (2018).
Boison, D. & Yegutkin, G. G. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36, 582–596 (2019).
During, M. J. & Spencer, D. D. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 32, 618–624 (1992).
Boison, D. Methylxanthines, seizures, and excitotoxicity. Handb. Exp. Pharmacol. 200, 251–266 (2011).
Aronica, E., Sandau, U. S., Iyer, A. & Boison, D. Glial adenosine kinase – a neuropathological marker of the epileptic brain. Neurochem. Int. 63, 688–695 (2013).
Li, T. et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J. Clin. Invest. 118, 571–582 (2008). This work unravelled a novel astrocyte-based mechanism of epileptogenesis, which forms the basis for the adenosine kinase hypothesis of epileptogenesis.
Williams-Karnesky, R. L. et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Invest. 123, 3552–3563 (2013). This work describes the discovery of a novel function of an isoform of adenosine kinase, which is expressed in the cell nucleus, as regulator of DNA methylation; therapeutic adenosine augmentation prevented maladaptive DNA methylation and thereby prevented epileptogenesis.
Boison, D. & Rho, J. M. Epigenetics and epilepsy prevention: the therapeutic potential of adenosine and metabolic therapies. Neuropharmacology 167, 107741 (2020).
Gouder, N., Fritschy, J.-M. & Boison, D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia 44, 877–885 (2003).
Sandhu, M. R. S. et al. Astroglial glutamine synthetase and the pathogenesis of mesial temporal lobe epilepsy. Front. Neurol. 12, 665334 (2021).
Peterson, A. R. & Binder, D. K. Astrocyte glutamate uptake and signaling as novel targets for antiepileptogenic therapy. Front. Neurol. 11, 1006 (2020).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997). This seminal study provides the first direct evidence that dysregulation of astrocytic glutamate homeostasis has a key role in epilepsy and its development.
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
Sada, N., Lee, S., Katsu, T., Otsuki, T. & Inoue, T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347, 1362–1367 (2015). This landmark study identified lactate dehydrogenase as a major metabolic component and therapeutic target for the treatment of epilepsy.
Muraleedharan, R. et al. AMPK-regulated astrocytic lactate shuttle plays a non-cell-autonomous role in neuronal survival. Cell Rep. 32, 108092 (2020).
Kobow, K. & Blumcke, I. The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia 52, 15–19 (2011).
Miller-Delaney, S. F. C. et al. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 138, 616–631 (2015).
Martins-Ferreira, R. et al. Epilepsy progression is associated with cumulative DNA methylation changes in inflammatory genes. Prog. Neurobiol. 209, 102207 (2022).
Boison, D. et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc. Natl Acad. Sci. USA 99, 6985–6990 (2002).
Simeone, T. A., Simeone, K. A., Stafstrom, C. E. & Rho, J. M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133, 233–241 (2018).
deCampo, D. M. & Kossoff, E. H. Ketogenic dietary therapies for epilepsy and beyond. Curr. Opin. Clin. Nutr. Metab. Care 22, 264–268 (2019).
Ockuly, J. C. et al. Behavioral, cognitive, and safety profile of 2-deoxy-2-glucose (2DG) in adult rats. Epilepsy Res. 101, 246–252 (2012).
Stafstrom, C. E. et al. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann. Neurol. 65, 435–447 (2009).
Pan, Y.-Z., Sutula, T. P. & Rutecki, P. A. 2-Deoxy-d-glucose reduces epileptiform activity by presynaptic mechanisms. J. Neurophysiol. 121, 1092–1101 (2019).
Koenig, J. B. et al. Glycolytic inhibitor 2-deoxyglucose prevents cortical hyperexcitability after traumatic brain injury. JCI Insight 5, e126506 (2019).
Lusardi, T. A. et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99, 500–509 (2015).
Masino, S. A. et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Invest. 121, 2679–2683 (2011). This study identified a novel mechanism through which a high-fat, low-carbohydrate metabolic treatment (ketogenic diet) prevents seizures in epilepsy.
Li, T. et al. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain 130, 1276–1288 (2007).
Li, T., Ren, G., Kaplan, D. L. & Boison, D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 84, 238–241 (2009).
Sandau, U. S. et al. Transient use of a systemic adenosine kinase inhibitor attenuates epilepsy development in mice. Epilepsia 60, 615–625 (2019).
Diamond, M. L. et al. Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia 56, 1198–1206 (2015).
Zimmer, T. S. et al. Tuberous sclerosis complex as disease model for investigating mTOR-related gliopathy during epileptogenesis. Front. Neurol. 11, 1028 (2020).
Eyo, U. B., Murugan, M. & Wu, L.-J. Microglia–neuron communication in epilepsy. Glia 65, 5–18 (2017).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). This study shows that reactive microglia secrete inflammatory cytokines and thereby induce dysfunction in astrocytes, which lose their homeostatic and cell survival functions, while inducing the death of neurons and oligodendrocytes.
Giannoni, P. et al. The pericyte–glia interface at the blood–brain barrier. Clin. Sci. 132, 361–374 (2018).
Taylor, X. et al. Activated endothelial cells induce a distinct type of astrocytic reactivity. Commun. Biol. 5, 282 (2022). This study shows that a subtype of neurotoxic astrocytes with a distinctive extracellular matrix remodelling profile is induced by activated endothelial cells and is distinct from astrocytes that are activated by microglia.
Varvel, N. H. et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc. Natl Acad. Sci. USA 113, E5665–E5674 (2016).
Xanthos, D. N. & Sandkuhler, J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 15, 43–53 (2014).
Maroso, M. et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 16, 413–419 (2010).
Zurolo, E. et al. Activation of TLR, RAGE and HMGB1 signaling in malformations of cortical development. Brain 134, 1015–1032 (2011).
Fields, R. D. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron–glia signaling. Semin. Cell Dev. Biol. 22, 214–219 (2011).
Rojas, A., Chen, D., Ganesh, T., Varvel, N. H. & Dingledine, R. The COX-2/prostanoid signaling cascades in seizure disorders. Expert. Opin. Ther. Targets 23, 1–13 (2019).
Gruber, V.-E. et al. Increased expression of complement components in tuberous sclerosis complex and focal cortical dysplasia type 2B brain lesions. Epilepsia 63, 364–374 (2022).
Benson, M. J. et al. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol. Dis. 76, 87–97 (2015).
Vezzani, A., Maroso, M., Balosso, S., Sanchez, M. A. & Bartfai, T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 25, 1281–1289 (2011).
Binder, D. K. & Steinhauser, C. Functional changes in astroglial cells in epilepsy. Glia 54, 358–368 (2006).
Vezzani, A. & Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82 (2015).
Khan, D. et al. Experimental febrile seizures impair interastrocytic gap junction coupling in juvenile mice. J. Neurosci. Res. 94, 804–813 (2016).
Deshpande, T. et al. Constitutive deletion of astrocytic connexins aggravates kainate-induced epilepsy. Glia 68, 2136–2147 (2020).
Löscher, W. & Friedman, A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: a cause, consequence, or both?. Int. J. Mol. Sci. 21, 591 (2020).
van Lanen, R. H. et al. Microvascular changes associated with epilepsy: a narrative review. J. Cereb. Blood Flow. Metab. 41, 2492–2509 (2021).
Yamanaka, G. et al. The neuroinflammatory role of pericytes in epilepsy. Biomedicines 9, 759 (2021).
Deshpande, T. et al. Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 65, 1809–1820 (2017).
Braganza, O. et al. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 53, 1898–1906 (2012).
Ralay Ranaivo, H. & Wainwright, M. S. Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res. 1313, 222–231 (2010).
Henning, L., Steinhäuser, C. & Bedner, P. Initiation of experimental temporal lobe epilepsy by early astrocyte uncoupling is independent of TGFβR1/ALK5 signaling. Front. Neurol. 12, 660591 (2021).
Ralay Ranaivo, H., Hodge, J. N., Choi, N. & Wainwright, M. S. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J. Neuroinflammation 9, 68 (2012).
Korotkov, A. et al. Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of microRNA-155 in cultured human astrocytes. J. Neuroinflammation 15, 211 (2018).
Broekaart, D. W. et al. The matrix metalloproteinase inhibitor IPR-179 has antiseizure and antiepileptogenic effects. J. Clin. Invest. 131, 138332 (2021). This study shows that increased expression of MMPs in astrocytes is a prominent hallmark of the human epileptogenic brain and that MMP inhibition mediates antiseizure and antiepileptogenic effects in rodent epilepsy models and attenuates seizure-induced cognitive decline.
Twible, C., Abdo, R. & Zhang, Q. Astrocyte role in temporal lobe epilepsy and development of mossy fiber sprouting. Front. Cell Neurosci. 15, 725693 (2021).
Rankin-Gee, E. K. et al. Perineuronal net degradation in epilepsy. Epilepsia 56, 1124–1133 (2015).
Kim, S. Y. et al. TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci. Rep. 7, 7711 (2017).
Pollock, E., Everest, M., Brown, A. & Poulter, M. O. Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol. Dis. 70, 21–31 (2014).
Chaunsali, L., Tewari, B. P. & Sontheimer, H. Perineuronal net dynamics in the pathophysiology of epilepsy. Epilepsy Curr. 21, 273–281 (2021).
Parsons, A. L. M. et al. The interconnected mechanisms of oxidative stress and neuroinflammation in epilepsy. Antioxidants 11, 157 (2022).
Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62, 649–671 (2000).
Pearson-Smith, J. N. & Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 18, 2365 (2017).
Terrone, G., Balosso, S., Pauletti, A., Ravizza, T. & Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 167, 107742 (2020). This review discusses evidence for disease modifications mediated by combinations of anti-inflammatory and antioxidant treatments in animal models of acquired epilepsy.
Kovac, S., Domijan, A.-M., Walker, M. C. & Abramov, A. Y. Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 5, e1442 (2014).
Habas, A., Hahn, J., Wang, X. & Margeta, M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc. Natl Acad. Sci. USA 110, 18291–18296 (2013).
Patel, D. C., Tewari, B. P., Chaunsali, L. & Sontheimer, H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 20, 282–297 (2019).
Sørensen, M. F. et al. High expression of cystine–glutamate antiporter xCT (SLC7A11) is an independent biomarker for epileptic seizures at diagnosis in glioma. J. Neurooncol. 138, 49–53 (2018).
Alcoreza, O., Jagarlamudi, S., Savoia, A., Campbell, S. L. & Sontheimer, H. Sulfasalazine decreases astrogliosis-mediated seizure burden. Epilepsia 63, 844–854 (2022).
Singh, N. et al. Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 20, 1324–1363 (2014).
Chen, S. et al. Iron metabolism and ferroptosis in epilepsy. Front. Neurosci. 14, 601193 (2020).
Zimmer, T. S. et al. Chronic activation of anti-oxidant pathways and iron accumulation in epileptogenic malformations. Neuropathol. Appl. Neurobiol. 46, 546–563 (2020).
Zimmer, T. S. et al. Seizure-mediated iron accumulation and dysregulated iron metabolism after status epilepticus and in temporal lobe epilepsy. Acta Neuropathol. 142, 729–759 (2021). This study suggests that seizure-mediated, chronic neuronal iron uptake has a role in neuronal dysfunction and loss in TLE with hippocampal sclerosis.
Löscher, W., Potschka, H., Sisodiya, S. M. & Vezzani, A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 72, 606–638 (2020).
Weidner, L. D. et al. The expression of inflammatory markers and their potential influence on efflux transporters in drug-resistant mesial temporal lobe epilepsy tissue. Epilepsia 59, 1507–1517 (2018).
Potschka, H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia 51, 1333–1347 (2010).
Terrone, G., Frigerio, F., Balosso, S., Ravizza, T. & Vezzani, A. Inflammation and reactive oxygen species in status epilepticus: biomarkers and implications for therapy. Epilepsy Behav. 101, 106275 (2019).
Lai, Y.-C. et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann. Clin. Transl. Neurol. 7, 2467–2474 (2020).
Harris, J. L., Choi, I.-Y. & Brooks, W. M. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 7, 202 (2015).
Matsuo, N. et al. The correlation between 1H-MR spectroscopy and clinical manifestation with tuberous sclerosis complex. Neuropediatrics 38, 126–129 (2007).
Hammen, T. et al. Non-invasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed. 21, 545–552 (2008).
Turkdogan-Sozuer, D., Ozek, M. M., Sav, A., Dincer, A. & Pamir, M. N. Serial MRI and MRS studies with unusual findings in Rasmussen’s encephalitis. Eur. Radiol. 10, 962–966 (2000).
Obenaus, A. Neuroimaging biomarkers for epilepsy: advances and relevance to glial cells. Neurochem. Int. 63, 712–718 (2013).
Kumlien, E., Hilton-Brown, P., Spannare, B. & Gillberg, P. G. In vitro quantitative autoradiography of [3H]-L-deprenyl and [3H]-PK11195 binding sites in human epileptic hippocampus. Epilepsia 33, 610–617 (1992).
Kumlien, E., Nilsson, A., Hagberg, G., Langstrom, B. & Bergstrom, B. PET with 11C-deuterium-deprenyl and 18F-FDG in focal epilepsy. Acta Neurol. Scand. 103, 360–366 (2001).
Kumlien, E. et al. Positron emission tomography with [11C]deuterium-deprenyl in temporal lobe epilepsy. Epilepsia 36, 712–721 (1995). This study is the first to demonstrate the clinical application of PET to the imaging of astrocytes in patients with epilepsy.
Buck, A. et al. Monoamine oxidase B single-photon emission tomography with [123]Ro 43-0463: imaging in volunteers and patients with temporal lobe epilepsy. Eur. J. Nucl. Med. 25, 464–470 (1998).
Harada, R. et al. Imaging of reactive astrogliosis by positron emission tomography. Front. Neurosci. 16, 807435 (2022). An extensive overview of the tools available for imaging reactive astrogliosis using PET tracers in the clinical setting.
Regunathan, S., Feinstein, D. L. & Reis, D. J. Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J. Neurosci. Res. 34, 681–688 (1993).
Olmos, G., Alemany, R., Escriba, P. V. & García-Sevilla, J. A. The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br. J. Pharmacol. 111, 997–1002 (1994).
Patodia, S. et al. Adenosine kinase and adenosine receptors A1 R and A2A R in temporal lobe epilepsy and hippocampal sclerosis and association with risk factors for SUDEP. Epilepsia 61, 787–797 (2020).
Matthews, P. M. & Datta, G. Positron-emission tomography molecular imaging of glia and myelin in drug discovery for multiple sclerosis. Expert. Opin. Drug Discov. 10, 557–570 (2015).
Wilson, H. et al. Imidazoline 2 binding sites reflecting astroglia pathology in Parkinson’s disease: an in vivo 11C-BU99008 PET study. Brain 142, 3116–3128 (2019).
Sun, M.-J., Liu, F., Zhao, Y.-F. & Wu, X.-A. In vivo positron emission tomography imaging of adenosine A2A receptors. Front. Pharmacol. 11, 599857 (2020).
Wu, Y. et al. Metabolic changes in early poststatus epilepticus measured by MR spectroscopy in rats. J. Cereb. Blood Flow. Metab. 35, 1862–1870 (2015).
Filibian, M. et al. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 53, 1907–1916 (2012).
Motamedi, G. & Meador, K. Epilepsy and cognition. Epilepsy Behav. 4, S25–S38 (2003).
Pascente, R. et al. Cognitive deficits and brain myo-inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis. 93, 146–155 (2016). This article demonstrates that hippocampal myo-inositol levels measured with 1H-MRS is a prognostic biomarker of epileptogenesis in experimental models.
Bascuñana, P. et al. Ex vivo characterization of neuroinflammatory and neuroreceptor changes during epileptogenesis using candidate positron emission tomography biomarkers. Epilepsia 60, 2325–2333 (2019).
Chen, S.-Q. et al. 1H-MRS evaluation of therapeutic effect of neural stem cell transplantation on Alzheimer’s disease in AβPP/PS1 double transgenic mice. J. Alzheimers Dis. 28, 71–80 (2012).
Gurnett, C. A., Landt, M. & Wong, M. Analysis of cerebrospinal fluid glial fibrillary acidic protein after seizures in children. Epilepsia 44, 1455–1458 (2003).
Steinhoff, B. J. et al. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res. 36, 75–82 (1999).
Simani, L., Elmi, M. & Asadollahi, M. Serum GFAP level: a novel adjunctive diagnostic test in differentiate epileptic seizures from psychogenic attacks. Seizure 61, 41–44 (2018). This study provides clinical evidence that serum levels of GFAP can distinguish epileptic seizures from psychogenic seizures.
Elhady, M. et al. Circulating glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase-L1 as markers of neuronal damage in children with epileptic seizures. Childs Nerv. Syst. 37, 879–884 (2021).
Savaş, M. et al. Glial fibrillary acidic protein (GFAP)-antibody in children with focal seizures of undetermined cause. Acta Neurol. Belg. 121, 1275–1280 (2021).
Liang, K.-G. et al. Increased serum S100B levels in patients with epilepsy: a systematic review and meta-analysis study. Front. Neurosci. 13, 456 (2019). This meta-analysis demonstrates that blood levels of S100β are increased in patients with epilepsy compared with healthy controls.
Lu, C. et al. Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: a case-control study. Neurosci. Lett. 484, 139–142 (2010).
Moshrefi-Ravasdjani, B. et al. Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct. Funct. 222, 831–847 (2017).
Galanopoulou, A. S., French, J. A., O’Brien, T. & Simonato, M. Harmonization in preclinical epilepsy research: a joint AES/ILAE translational initiative. Epilepsia 58, 7–9 (2017).
Akin, D. et al. IL-1β is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence. Neurobiol. Dis. 44, 259–269 (2011).
Thompson, J. A. et al. Astrocyte reactivity in a mouse model of SCN8A epileptic encephalopathy. Epilepsia Open 7, 280–292 (2022).
Lahuerta, M. et al. Reactive glia-derived neuroinflammation: a novel hallmark in Lafora progressive myoclonus epilepsy that progresses with age. Mol. Neurobiol. 57, 1607–1621 (2020).
Arena, A. et al. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence. Brain Pathol. 29, 351–365 (2019).
Jiang, J. & Dingledine, R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol. Sci. 34, 413–423 (2013).
Borodinova, A. A., Balaban, P. M., Bezprozvanny, I. B., Salmina, A. B. & Vlasova, O. L. Genetic constructs for the control of astrocytes’ activity. Cells 10, 1600 (2021).
Duarte, F. & Déglon, N. Genome editing for CNS disorders. Front. Neurosci. 14, 579062 (2020).
Bradley, S. J., Tobin, A. B. & Prihandoko, R. The use of chemogenetic approaches to study the physiological roles of muscarinic acetylcholine receptors in the central nervous system. Neuropharmacology 136, 421–426 (2018).
Lee, H.-G., Wheeler, M. A. & Quintana, F. J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 21, 339–358 (2022).
Magistretti, P. J. & Pellerin, L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol. Psychiatry 1, 445–452 (1996).
Garcia-Hernandez, R. et al. Mapping microglia and astrocytes in vivo using diffusion MRI. Sci. Adv. 8, eabq2923 (2022).
Takata, N. et al. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia 66, 2013–2023 (2018).
McTague, A., Howell, K. B., Cross, J. H., Kurian, M. A. & Scheffer, I. E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 15, 304–316 (2016).
Northrup, H. et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr. Neurol. 123, 50–66 (2021).
Kondo, T. et al. Modeling Alexander disease with patient iPSCs reveals cellular and molecular pathology of astrocytes. Acta Neuropathol. Commun. 4, 69 (2016).
Sase, S., Takanohashi, A., Vanderver, A. & Almad, A. Astrocytes, an active player in Aicardi-Goutières syndrome. Brain Pathol. 28, 399–407 (2018).
van Heteren, J. T. et al. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutières syndrome. Glia 56, 568–578 (2008).
Acknowledgements
The authors gratefully acknowledge their sources of support: Era-Net Neuron Ebio2, and American Epilepsy Society Seed Grant and NORSE Institute (A.V.); the Associazione Italiana Contro l’Epilessia (AICE-FIRE) (T.R.); and the National Institutes of Health (NIH grants NS103740, NS065957, NS127846), the Office of the Assistant Secretary of Defense for Health Affairs through the Epilepsy Research Program under award no. W81XWH2210638, and a Catalyst Award from CURE Epilepsy (D.B.); grants from the EU (H2020-MSCA-ITN project no. 722053 EU-GliaPhD) and BMBF (16GW0182 CONNEXIN, 01DN20001 CONNEX) (C.S.); and grants from the EU (H2020-MSCA-ITN project no. 722053 EU-GliaPhD), H2020-Twinning project EpiEpiNet (no. 952455) and the Dutch Organization for Medical Sciences (ZonMw) (E.A.).
Author information
Authors and Affiliations
Contributions
A.V. supervised the manuscript preparation, wrote the Introduction and Conclusions and, with E.A., wrote the section ‘Immune and inflammatory functions’. E.A. wrote Box 1. P.B. and C.S. wrote the section ‘Gliotransmission’. D.B. wrote the section ‘Cell metabolism’. T.R. wrote the section ‘In vivo monitoring of astrocyte reactivity’ and prepared Box 2. All the authors contributed to preparing Box 3 and the figures, and contributed equally to revising and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks T. Eid, M. Okada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed for peer-reviewed publications published between January 1990 and April 2022 with the term “astrocytes”. We then refined our search terms to be “astrocytes” AND (as individual combinatory terms) “biomarker(s)”, “epilepsy”, “epileptogenesis”, “gliotransmission”, “imaging”, “metabolism”, “neurobiology”, “(neuro)inflammation”, “seizure(s)” and “treatment(s)”. Selection criteria applied to full-text outputs were the novelty of study findings and their relevance to neurologists; inclusion was decided collectively by all authors. One relevant historical reference outside the search time frame was also included.
Glossary
- Tripartite synapse
-
The association of and communication between the traditional bipartite synapse and surrounding astrocytes that contributes to synaptic transmission.
- Paroxysmal depolarization shifts
-
Abnormally prolonged depolarizations with superimposed action potentials in neurons in epileptic foci; the cellular basis of interictal epileptic spikes in the electroencephalogram.
- Gap junction coupling
-
Cell-to-cell transfer of ions and small molecules mediated by gap junction channels.
- Luciferin–luciferase assay
-
An assay for quantitative detection of ATP, based on the ability of the firefly enzyme luciferase to convert luciferin into oxyluciferin in an ATP-dependent reaction that generates light.
- Kindling
-
A model of epilepsy in which repeated electrical or chemical stimulation leads to a persistent decrease in seizure threshold.
- Redox proteome
-
Proteins that undergo redox modifications during oxidative stress.
- NG2 glia
-
Glial cells that express nerve/glial antigen 2 and that differentiate into oligodendrocytes in the white matter, but mostly keep the NG2 phenotype throughout adulthood in the grey matter; their function is under debate.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vezzani, A., Ravizza, T., Bedner, P. et al. Astrocytes in the initiation and progression of epilepsy. Nat Rev Neurol 18, 707–722 (2022). https://doi.org/10.1038/s41582-022-00727-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00727-5
This article is cited by
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment
Nature Reviews Neuroscience (2024)
-
Identification of gene regulatory networks affected across drug-resistant epilepsies
Nature Communications (2024)
-
Downregulation of Ubiquitin-Specific Protease 15 (USP15) Does Not Provide Therapeutic Benefit in Experimental Mesial Temporal Lobe Epilepsy
Molecular Neurobiology (2024)
-
Astroglial calcium signaling and homeostasis in tuberous sclerosis complex
Acta Neuropathologica (2024)
-
Is tuberous sclerosis complex-associated autism a preventable and treatable disorder?
World Journal of Pediatrics (2024)