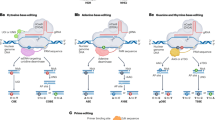
Abstract
DNA methylation, also known as 5-methylcytosine, is an epigenetic modification that has crucial functions in plant growth, development and adaptation. The cellular DNA methylation level is tightly regulated by the combined action of DNA methyltransferases and demethylases. Protein complexes involved in the targeting and interpretation of DNA methylation have been identified, revealing intriguing roles of methyl-DNA binding proteins and molecular chaperones. Structural studies and in vitro reconstituted enzymatic systems have provided mechanistic insights into RNA-directed DNA methylation, the main pathway catalysing de novo methylation in plants. A better understanding of the regulatory mechanisms will enable locus-specific manipulation of the DNA methylation status. CRISPR–dCas9-based epigenome editing tools are being developed for this goal. Given that DNA methylation patterns can be stably transmitted through meiosis, and that large phenotypic variations can be contributed by epimutations, epigenome editing holds great promise in crop breeding by creating additional phenotypic variability on the same genetic material.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Alleman, M. et al. An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442, 295–298 (2006).
He, L. et al. DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat. Commun. 13, 1335 (2022).
Schmitz, R. J., Lewis, Z. A. & Goll, M. G. DNA methylation: shared and divergent features across eukaryotes. Trends Genet. 35, 818–827 (2019).
Wyatt, G. R. Occurrence of 5-methylcytosine in nucleic acids. Nature 166, 237–238 (1950).
Vidalis, A. et al. Methylome evolution in plants. Genome Biol. 17, 264 (2016).
Law, J. A. & Jacobsen, S. E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11, 204–220 (2010).
Stroud, H., Greenberg, M. V., Feng, S., Bernatavichute, Y. V. & Jacobsen, S. E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152, 352–364 (2013).
Stroud, H. et al. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat. Struct. Mol. Biol. 21, 64–72 (2014).
Matzke, M. A. & Mosher, R. A. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 15, 394–408 (2014).
Zhang, H., Gong, Z. & Zhu, J. K. Active DNA demethylation in plants: 20 years of discovery and beyond. J. Integr. Plant Biol. 64, 2217–2239 (2022).
Zhang, H. et al. Sulfamethazine suppresses epigenetic silencing in Arabidopsis by impairing folate synthesis. Plant Cell 24, 1230–1241 (2012).
Zhou, H. R. et al. Folate polyglutamylation is involved in chromatin silencing by maintaining global DNA methylation and histone H3K9 dimethylation in Arabidopsis. Plant Cell 25, 2545–2559 (2013).
Groth, M. et al. MTHFD1 controls DNA methylation in i. Nat. Commun. 7, 11640 (2016).
Gong, Z. et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111, 803–814 (2002).
Hollwey, E., Briffa, A., Howard, M. & Zilberman, D. Concepts, mechanisms and implications of long-term epigenetic inheritance. Curr. Opin. Genet. Dev. 81, 102087 (2023).
Skvortsova, K., Iovino, N. & Bogdanovic, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 19, 774–790 (2018).
Erdmann, R. M. & Picard, C. L. RNA-directed DNA methylation. PLoS Genet. 16, e1009034 (2020).
Matzke, M., Kanno, T., Daxinger, L., Huettel, B. & Matzke, A. J. RNA-mediated chromatin-based silencing in plants. Curr. Opin. Cell Biol. 21, 367–376 (2009).
Vaucheret, H. & Voinnet, O. The plant siRNA landscape. Plant Cell 36, 246–275 (2023).
Mari-Ordonez, A. et al. Reconstructing de novo silencing of an active plant retrotransposon. Nat. Genet. 45, 1029–1039 (2013).
Creasey, K. M. et al. miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature 508, 411–415 (2014).
Cuerda-Gil, D. & Slotkin, R. K. Non-canonical RNA-directed DNA methylation. Nat. Plants 2, 16163 (2016).
Zhang, H. et al. DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV. Proc. Natl Acad. Sci. USA 110, 8290–8295 (2013).
Law, J. A. et al. Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature 498, 385–389 (2013).
Wang, Y. et al. ZMP recruits and excludes Pol IV-mediated DNA methylation in a site-specific manner. Sci. Adv. 8, eadc9454 (2022).
Zhou, M., Palanca, A. M. S. & Law, J. A. Locus-specific control of the de novo DNA methylation pathway in Arabidopsis by the CLASSY family. Nat. Genet. 50, 865–873 (2018).
Zhou, M. et al. The CLASSY family controls tissue-specific DNA methylation patterns in Arabidopsis. Nat. Commun. 13, 244 (2022).
Huang, K. et al. Pol IV and RDR2: a two-RNA-polymerase machine that produces double-stranded RNA. Science 374, 1579–1586 (2021).
Mishra, V. et al. Assembly of a dsRNA synthesizing complex: RNA-DEPENDENT RNA POLYMERASE 2 contacts the largest subunit of NUCLEAR RNA POLYMERASE IV. Proc. Natl Acad. Sci. USA 118, e2019276118 (2021).
Singh, J., Mishra, V., Wang, F., Huang, H. Y. & Pikaard, C. S. Reaction mechanisms of Pol IV, RDR2, and DCL3 Drive RNA channeling in the siRNA-directed DNA methylation pathway. Mol. Cell 75, 576–589 e575 (2019).
Fukudome, A. et al. Structure and RNA template requirements of Arabidopsis RNA-DEPENDENT RNA POLYMERASE 2. Proc. Natl Acad. Sci. USA 118, e2115899118 (2021).
Nudler, E. RNA polymerase backtracking in gene regulation and genome instability. Cell 149, 1438–1445 (2012).
Yang, D. L. et al. DNA-dependent RNA polymerases in plants. Plant Cell 35, 3641–3661 (2023).
Li, S. et al. Detection of Pol IV/RDR2-dependent transcripts at the genomic scale in Arabidopsis reveals features and regulation of siRNA biogenesis. Genome Res. 25, 235–245 (2015).
Blevins, T. et al. Identification of Pol IV and RDR2-dependent precursors of 24 nt siRNAs guiding de novo DNA methylation in Arabidopsis. eLife 4, e09591 (2015).
Zhai, J. et al. A one precursor one siRNA model for pol IV-dependent siRNA biogenesis. Cell 163, 445–455 (2015).
Yang, D. L. et al. Dicer-independent RNA-directed DNA methylation in Arabidopsis. Cell Res. 26, 66–82 (2016).
Ye, R. et al. A dicer-independent route for biogenesis of siRNAs that direct DNA methylation in Arabidopsis. Mol. Cell 61, 222–235 (2016).
Haag, J. R. et al. In vitro transcription activities of Pol IV, Pol V, and RDR2 reveal coupling of Pol IV and RDR2 for dsRNA synthesis in plant RNA silencing. Mol. Cell 48, 811–818 (2012).
Nagano, H., Fukudome, A., Hiraguri, A., Moriyama, H. & Fukuhara, T. Distinct substrate specificities of Arabidopsis DCL3 and DCL4. Nucleic Acids Res. 42, 1845–1856 (2014).
Loffer, A. et al. A DCL3 dicing code within Pol IV-RDR2 transcripts diversifies the siRNA pool guiding RNA-directed DNA methylation. eLife 11, e73260 (2022).
Wang, Q. et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science 374, 1152–1157 (2021).
Wierzbicki, A. T., Haag, J. R. & Pikaard, C. S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135, 635–648 (2008).
Wierzbicki, A. T., Blevins, T. & Swiezewski, S. Long noncoding RNAs in plants. Annu. Rev. Plant Biol. 72, 245–271 (2021).
Liu, Z. W. et al. The SET domain proteins SUVH2 and SUVH9 are required for Pol V occupancy at RNA-directed DNA methylation loci. PLoS Genet. 10, e1003948 (2014).
Johnson, L. M. et al. SRA- and SET-domain-containing proteins link RNA polymerase V occupancy to DNA methylation. Nature 507, 124–128 (2014).
Zhou, J. X. et al. FVE promotes RNA-directed DNA methylation by facilitating the association of RNA polymerase V with chromatin. Plant J. 107, 467–479 (2021).
Huang, P. et al. MSI4/FVE is required for accumulation of 24-nt siRNAs and DNA methylation at a subset of target regions of RNA-directed DNA methylation. Plant J. 108, 347–357 (2021).
Yang, R. et al. The developmental regulator PKL is required to maintain correct DNA methylation patterns at RNA-directed DNA methylation loci. Genome Biol. 18, 103 (2017).
Niu, Q. et al. A histone H3K4me1-specific binding protein is required for siRNA accumulation and DNA methylation at a subset of loci targeted by RNA-directed DNA methylation. Nat. Commun. 12, 3367 (2021).
Sigman, M. J. et al. An siRNA-guided ARGONAUTE protein directs RNA polymerase V to initiate DNA methylation. Nat. Plants 7, 1461–1474 (2021).
Tsuzuki, M. et al. Broad noncoding transcription suggests genome surveillance by RNA polymerase V. Proc. Natl Acad. Sci. USA 117, 30799–30804 (2020).
Xie, G. et al. Structure and mechanism of the plant RNA polymerase V. Science 379, 1209–1213 (2023).
Marasco, M., Li, W., Lynch, M. & Pikaard, C. S. Catalytic properties of RNA polymerases IV and V: accuracy, nucleotide incorporation and rNTP/dNTP discrimination. Nucleic Acids Res. 45, 11315–11326 (2017).
Wierzbicki, A. T., Ream, T. S., Haag, J. R. & Pikaard, C. S. RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat. Genet. 41, 630–634 (2009).
El-Shami, M. et al. Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes Dev. 21, 2539–2544 (2007).
He, X. J. et al. An effector of RNA-directed DNA methylation in arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell 137, 498–508 (2009).
Lahmy, S. et al. Evidence for ARGONAUTE4-DNA interactions in RNA-directed DNA methylation in plants. Genes Dev. 30, 2565–2570 (2016).
Zhang, H. W. et al. A cryo-EM structure of KTF1-bound polymerase V transcription elongation complex. Nat. Commun. 14, 3118 (2023).
Liu, W. et al. RNA-directed DNA methylation involves co-transcriptional small-RNA-guided slicing of polymerase V transcripts in Arabidopsis. Nat. Plants 4, 181–188 (2018).
Wang, F., Huang, H. Y., Huang, J., Singh, J. & Pikaard, C. S. Enzymatic reactions of AGO4 in RNA-directed DNA methylation: siRNA duplex loading, passenger strand elimination, target RNA slicing, and sliced target retention. Genes Dev. 37, 103–118 (2023).
Wang, F. & Axtell, M. J. AGO4 is specifically required for heterochromatic siRNA accumulation at Pol V-dependent loci in Arabidopsis thaliana. Plant J. 90, 37–47 (2017).
Zhong, X. et al. Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 157, 1050–1060 (2014).
Du, J., Johnson, L. M., Jacobsen, S. E. & Patel, D. J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 16, 519–532 (2015).
Zhang, H., Lang, Z. & Zhu, J. K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 19, 489–506 (2018).
Riggs, A. D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 14, 9–25 (1975).
Holliday, R. & Pugh, J. E. DNA modification mechanisms and gene activity during development. Science 187, 226–232 (1975).
Bashtrykov, P. et al. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem. Biol. 19, 572–578 (2012).
Song, J., Teplova, M., Ishibe-Murakami, S. & Patel, D. J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science 335, 709–712 (2012).
Adam, S., Klingel, V., Radde, N. E., Bashtrykov, P. & Jeltsch, A. On the accuracy of the epigenetic copy machine: comprehensive specificity analysis of the DNMT1 DNA methyltransferase. Nucleic Acids Res. 51, 6622–6633 (2023).
Bostick, M. et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 317, 1760–1764 (2007).
Sharif, J. et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 450, 908–912 (2007).
Woo, H. R., Pontes, O., Pikaard, C. S. & Richards, E. J. VIM1, a methylcytosine-binding protein required for centromeric heterochromatinization. Genes Dev. 21, 267–277 (2007).
Woo, H. R., Dittmer, T. A. & Richards, E. J. Three SRA-domain methylcytosine-binding proteins cooperate to maintain global CpG methylation and epigenetic silencing in Arabidopsis. PLoS Genet. 4, e1000156 (2008).
Petryk, N., Bultmann, S., Bartke, T. & Defossez, P. A. Staying true to yourself: mechanisms of DNA methylation maintenance in mammals. Nucleic Acids Res. 49, 3020–3032 (2021).
Ossowski, S. et al. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327, 92–94 (2010).
Schmitz, R. J. et al. Transgenerational epigenetic instability is a source of novel methylation variants. Science 334, 369–373 (2011).
Becker, C. et al. Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature 480, 245–249 (2011).
Shahryary, Y. et al. AlphaBeta: computational inference of epimutation rates and spectra from high-throughput DNA methylation data in plants. Genome Biol. 21, 260 (2020).
Denkena, J., Johannes, F. & Colome-Tatche, M. Region-level epimutation rates in Arabidopsis thaliana. Heredity 127, 190–202 (2021).
Tang, K., Zhu, X., Xie, S., Lang, Z. & Zhu, J. K. Transgenerational increases in DNA methylation in Arabidopsis plants defective in active DNA demethylation. Proc. Natl Acad. Sci. USA 121, e2320468121 (2024).
Leichter, S. M., Du, J. & Zhong, X. Structure and mechanism of plant DNA methyltransferases. Adv. Exp. Med. Biol. 1389, 137–157 (2022).
Vilkaitis, G., Suetake, I., Klimasauskas, S. & Tajima, S. Processive methylation of hemimethylated CpG sites by mouse Dnmt1 DNA methyltransferase. J. Biol. Chem. 280, 64–72 (2005).
Goyal, R., Reinhardt, R. & Jeltsch, A. Accuracy of DNA methylation pattern preservation by the Dnmt1 methyltransferase. Nucleic Acids Res. 34, 1182–1188 (2006).
Jeltsch, A. & Jurkowska, R. Z. Allosteric control of mammalian DNA methyltransferases - a new regulatory paradigm. Nucleic Acids Res. 44, 8556–8575 (2016).
Briffa, A. et al. Millennia-long epigenetic fluctuations generate intragenic DNA methylation variance in Arabidopsis populations. Cell Syst. 14, 953–967 e917 (2023).
Wang, Q. et al. Imprecise DNMT1 activity coupled with neighbor-guided correction enables robust yet flexible epigenetic inheritance. Nat. Genet. 52, 828–839 (2020).
Zhu, J. K. Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 43, 143–166 (2009).
Morales-Ruiz, T. et al. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl Acad. Sci. USA 103, 6853–6858 (2006).
Ortega-Galisteo, A. P., Morales-Ruiz, T., Ariza, R. R. & Roldan-Arjona, T. Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol. Biol. 67, 671–681 (2008).
Gehring, M. et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124, 495–506 (2006).
Du, X. et al. Molecular basis of the plant ROS1-mediated active DNA demethylation. Nat. Plants 9, 271–279 (2023).
Ponferrada-Marin, M. I., Roldan-Arjona, T. & Ariza, R. R. ROS1 5-methylcytosine DNA glycosylase is a slow-turnover catalyst that initiates DNA demethylation in a distributive fashion. Nucleic Acids Res. 37, 4264–4274 (2009).
Scharer, O. D. & Jiricny, J. Recent progress in the biology, chemistry and structural biology of DNA glycosylases. Bioessays 23, 270–281 (2001).
Tang, K., Lang, Z., Zhang, H. & Zhu, J. K. The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2, 16169 (2016).
Qian, W. et al. A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science 336, 1445–1448 (2012).
Nie, W. F. et al. Histone acetylation recruits the SWR1 complex to regulate active DNA demethylation in Arabidopsis. Proc. Natl Acad. Sci. USA 116, 16641–16650 (2019).
Liu, P. et al. A novel protein complex that regulates active DNA demethylation in Arabidopsis. J. Integr. Plant Biol. 63, 772–786 (2021).
Lei, M. et al. Regulatory link between DNA methylation and active demethylation in Arabidopsis. Proc. Natl Acad. Sci. USA 112, 3553–3557 (2015).
Williams, B. P., Pignatta, D., Henikoff, S. & Gehring, M. Methylation-sensitive expression of a DNA demethylase gene serves as an epigenetic rheostat. PLoS Genet. 11, e1005142 (2015).
Williams, B. P. & Gehring, M. Stable transgenerational epigenetic inheritance requires a DNA methylation-sensing circuit. Nat. Commun. 8, 2124 (2017).
Doerfler, W. DNA methylation and gene activity. Annu. Rev. Biochem. 52, 93–124 (1983).
Weber, H. & Graessmann, A. Biological-activity of hemimethylated and single-stranded-DNA after direct gene-transfer into tobacco protoplasts. FEBS Lett. 253, 163–166 (1989).
Kass, S. U., Landsberger, N. & Wolffe, A. P. DNA methylation directs a time-dependent repression of transcription initiation. Curr. Biol. 7, 157–165 (1997).
Buschhausen, G., Wittig, B., Graessmann, M. & Graessmann, A. Chromatin structure is required to block transcription of the methylated herpes simplex virus thymidine kinase gene. Proc. Natl Acad. Sci. USA 84, 1177–1181 (1987).
Boyes, J. & Bird, A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell 64, 1123–1134 (1991).
Liu, K., Shimbo, T., Song, X., Wade, P. A. & Min, J. Proteins that read DNA methylation. Adv. Exp. Med. Biol. 1389, 269–293 (2022).
Feng, Z. et al. Genetic analysis implicates a molecular chaperone complex in regulating epigenetic silencing of methylated genomic regions. J. Integr. Plant Biol. 63, 1451–1461 (2021).
Lang, Z. et al. The methyl-CpG-binding protein MBD7 facilitates active DNA demethylation to limit DNA hyper-methylation and transcriptional gene silencing. Mol. Cell 57, 971–983 (2015).
Qian, W. et al. Regulation of active DNA demethylation by an alpha-crystallin domain protein in Arabidopsis. Mol. Cell 55, 361–371 (2014).
Boone, B. A. et al. ACD15, ACD21, and SLN regulate the accumulation and mobility of MBD6 to silence genes and transposable elements. Sci. Adv. 9, eadi9036 (2023).
Crisp, P. A. et al. Stable unmethylated DNA demarcates expressed genes and their cis-regulatory space in plant genomes. Proc. Natl Acad. Sci. USA 117, 23991–24000 (2020).
Zemach, A. & Grafi, G. Characterization of Arabidopsis thaliana methyl-CpG-binding domain (MBD) proteins. Plant J. 34, 565–572 (2003).
Ito, M., Koike, A., Koizumi, N. & Sano, H. Methylated DNA-binding proteins from Arabidopsis. Plant Physiol. 133, 1747–1754 (2003).
Wu, Z. et al. Family-wide characterization of methylated DNA binding ability of Arabidopsis MBDs. J. Mol. Biol. 434, 167404 (2022).
Wang, S. et al. MBD2 couples DNA methylation to transposable element silencing during male gametogenesis. Nat. Plants 10, 13–24 (2024).
Ichino, L. et al. MBD5 and MBD6 couple DNA methylation to gene silencing through the J-domain protein SILENZIO. Science, https://doi.org/10.1126/science.abg6130 (2021).
Li, D. et al. The MBD7 complex promotes expression of methylated transgenes without significantly altering their methylation status. eLife 6, e19893 (2017).
Jackson, J. P., Lindroth, A. M., Cao, X. & Jacobsen, S. E. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416, 556–560 (2002).
Ebbs, M. L., Bartee, L. & Bender, J. H3 lysine 9 methylation is maintained on a transcribed inverted repeat by combined action of SUVH6 and SUVH4 methyltransferases. Mol. Cell Biol. 25, 10507–10515 (2005).
Ebbs, M. L. & Bender, J. Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18, 1166–1176 (2006).
Zhao, Q. Q., Lin, R. N., Li, L., Chen, S. & He, X. J. A methylated-DNA-binding complex required for plant development mediates transcriptional activation of promoter methylated genes. J. Integr. Plant Biol. 61, 120–139 (2019).
Xiao, X. et al. A group of SUVH methyl-DNA binding proteins regulate expression of the DNA demethylase ROS1 in Arabidopsis. J. Integr. Plant Biol. 61, 110–119 (2019).
Harris, C. J. et al. A DNA methylation reader complex that enhances gene transcription. Science 362, 1182–1186 (2018).
Niederhuth, C. E. et al. Widespread natural variation of DNA methylation within angiosperms. Genome Biol. 17, 194 (2016).
Sequeira-Mendes, J. et al. The functional topography of the arabidopsis genome is organized in a reduced number of linear motifs of chromatin states. Plant Cell 26, 2351–2366 (2014).
Jamge, B. et al. Histone variants shape chromatin states in Arabidopsis. eLife 12, e87714 (2023).
Zhao, L. et al. DNA methylation underpins the epigenomic landscape regulating genome transcription in Arabidopsis. Genome Biol. 23, 197 (2022).
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 (2012).
Illingworth, R. S. & Bird, A. P. CpG islands – ‘a rough guide’. FEBS Lett. 583, 1713–1720 (2009).
Bewick, A. J. & Schmitz, R. J. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 36, 103–110 (2017).
Gent, J. I. et al. CHH islands: de novo DNA methylation in near-gene chromatin regulation in maize. Genome Res. 23, 628–637 (2013).
Li, Q. et al. RNA-directed DNA methylation enforces boundaries between heterochromatin and euchromatin in the maize genome. Proc. Natl Acad. Sci. USA 112, 14728–14733 (2015).
Duan, C. G. et al. A pair of transposon-derived proteins function in a histone acetyltransferase complex for active DNA demethylation. Cell Res. 27, 226–240 (2017).
Haslbeck, M., Weinkauf, S. & Buchner, J. Small heat shock proteins: simplicity meets complexity. J. Biol. Chem. 294, 2121–2132 (2019).
Lang, Z. et al. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc. Natl Acad. Sci. USA 114, E4511–E4519 (2017).
Smith, J., Sen, S., Weeks, R. J., Eccles, M. R. & Chatterjee, A. Promoter DNA hypermethylation and paradoxical gene activation. Trends Cancer 6, 392–406 (2020).
Makarevich, G., Villar, C. B., Erilova, A. & Kohler, C. Mechanism of PHERES1 imprinting in Arabidopsis. J. Cell Sci. 121, 906–912 (2008).
Kawakatsu, T. et al. Unique cell-type-specific patterns of DNA methylation in the root meristem. Nat. Plants 2, 16058 (2016).
Zhan, X., Lu, Y., Zhu, J. K. & Botella, J. R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 63, 3–33 (2021).
de Mendoza, A. et al. Large-scale manipulation of promoter DNA methylation reveals context-specific transcriptional responses and stability. Genome Biol. 23, 163 (2022).
Zhu, H., Wang, G. & Qian, J. Transcription factors as readers and effectors of DNA methylation. Nat. Rev. Genet. 17, 551–565 (2016).
Kaluscha, S. et al. Evidence that direct inhibition of transcription factor binding is the prevailing mode of gene and repeat repression by DNA methylation. Nat. Genet. 54, 1895–1906 (2022).
O’Malley, R. C. et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165, 1280–1292 (2016).
Li, M. et al. Double DAP-seq uncovered synergistic DNA binding of interacting bZIP transcription factors. Nat. Commun. 14, 2600 (2023).
Feng, S., Zhong, Z., Wang, M. & Jacobsen, S. E. Efficient and accurate determination of genome-wide DNA methylation patterns in Arabidopsis thaliana with enzymatic methyl sequencing. Epigenetics Chromatin 13, 42 (2020).
Dai, Q. et al. Ultrafast bisulfite sequencing detection of 5-methylcytosine in DNA and RNA. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02034-w (2024).
Le, N. T. et al. Epigenetic regulation of spurious transcription initiation in Arabidopsis. Nat. Commun. 11, 3224 (2020).
Dantas Machado, A. C. et al. Evolving insights on how cytosine methylation affects protein-DNA binding. Brief. Funct. Genomics 14, 61–73 (2015).
Kribelbauer, J. F., Lu, X. J., Rohs, R., Mann, R. S. & Bussemaker, H. J. Toward a mechanistic understanding of DNA methylation readout by transcription factors. J. Mol. Biol. 432, 1801–1815 (2020).
Wang, B. et al. Structural insights into target DNA recognition by R2R3-MYB transcription factors. Nucleic Acids Res. 48, 460–471 (2020).
Charvin, M. et al. Single-cytosine methylation at W-boxes repels binding of WRKY transcription factors through steric hindrance. Plant Physiol. 192, 77–84 (2023).
Mann, I. K. et al. CG methylated microarrays identify a novel methylated sequence bound by the CEBPB|ATF4 heterodimer that is active in vivo. Genome Res. 23, 988–997 (2013).
Le, T. N., Miyazaki, Y., Takuno, S. & Saze, H. Epigenetic regulation of intragenic transposable elements impacts gene transcription in Arabidopsis thaliana. Nucleic Acids Res. 43, 3911–3921 (2015).
Saze, H. Epigenetic regulation of intragenic transposable elements: a two-edged sword. J. Biochem. 164, 323–328 (2018).
Marand, A. P., Eveland, A. L., Kaufmann, K. & Springer, N. M. cis-regulatory elements in plant development, adaptation, and evolution. Annu. Rev. Plant Biol. 74, 111–137 (2023).
Duan, C. G. et al. A protein complex regulates RNA processing of intronic heterochromatin-containing genes in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E7377–E7384 (2017).
Wang, X. et al. RNA-binding protein regulates plant DNA methylation by controlling mRNA processing at the intronic heterochromatin-containing gene IBM1. Proc. Natl Acad. Sci. USA 110, 15467–15472 (2013).
Saze, H. et al. Mechanism for full-length RNA processing of Arabidopsis genes containing intragenic heterochromatin. Nat. Commun. 4, 2301 (2013).
Lei, M. et al. Arabidopsis EDM2 promotes IBM1 distal polyadenylation and regulates genome DNA methylation patterns. Proc. Natl Acad. Sci. USA 111, 527–532 (2014).
Miura, A. et al. An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J. 28, 1078–1086 (2009).
Zhang, Y. Z. et al. Genome-wide distribution and functions of the AAE complex in epigenetic regulation in Arabidopsis. J. Integr. Plant Biol. 63, 707–722 (2021).
West, P. T. et al. Genomic distribution of H3K9me2 and DNA methylation in a maize genome. PLoS ONE 9, e105267 (2014).
Espinas, N. A. et al. Transcriptional regulation of genes bearing intronic heterochromatin in the rice genome. PLoS Genet. 16, e1008637 (2020).
You, L. Y. et al. Intragenic heterochromatin-mediated alternative polyadenylation modulates miRNA and pollen development in rice. New Phytol. 232, 835–852 (2021).
Walker, J. et al. Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat. Genet. 50, 130–137 (2018).
Ong-Abdullah, M. et al. Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 525, 533–537 (2015).
Wang, X. et al. DNA methylation affects gene alternative splicing in plants: an example from rice. Mol. Plant. 9, 305–307 (2016).
Regulski, M. et al. The maize methylome influences mRNA splice sites and reveals widespread paramutation-like switches guided by small RNA. Genome Res. 23, 1651–1662 (2013).
Higo, A. et al. DNA methylation is reconfigured at the onset of reproduction in rice shoot apical meristem. Nat. Commun. 11, 4079 (2020).
Wang, L. et al. Reinforcement of CHH methylation through RNA-directed DNA methylation ensures sexual reproduction in rice. Plant Physiol. 188, 1189–1209 (2022).
She, W. et al. Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development 140, 4008–4019 (2013).
Ingouff, M. et al. Live-cell analysis of DNA methylation during sexual reproduction in Arabidopsis reveals context and sex-specific dynamics controlled by noncanonical RdDM. Genes Dev. 31, 72–83 (2017).
Borg, M. et al. Epigenetic reprogramming rewires transcription during the alternation of generations in Arabidopsis. eLife 10, e61894 (2021).
Hsieh, P. H. et al. Arabidopsis male sexual lineage exhibits more robust maintenance of CG methylation than somatic tissues. Proc. Natl Acad. Sci. USA 113, 15132–15137 (2016).
Schmidt, A., Schmid, M. W. & Grossniklaus, U. Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142, 229–241 (2015).
Lanfear, R. Do plants have a segregated germline? PLoS Biol. 16, e2005439 (2018).
She, W. & Baroux, C. Chromatin dynamics in pollen mother cells underpin a common scenario at the somatic-to-reproductive fate transition of both the male and female lineages in Arabidopsis. Front. Plant Sci. 6, 294 (2015).
Yang, W. C., Ye, D., Xu, J. & Sundaresan, V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13, 2108–2117 (1999).
Schiefthaler, U. et al. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 96, 11664–11669 (1999).
Mendes, M. A. et al. The RNA-dependent DNA methylation pathway is required to restrict SPOROCYTELESS/NOZZLE expression to specify a single female germ cell precursor in Arabidopsis. Development 147, dev194274 (2020).
Olmedo-Monfil, V. et al. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464, 628–632 (2010).
Hernandez-Lagana, E., Rodriguez-Leal, D., Lua, J. & Vielle-Calzada, J. P. A multigenic network of ARGONAUTE4 clade members controls early megaspore formation in Arabidopsis. Genetics 204, 1045–1056 (2016).
Li, L., Wu, W., Zhao, Y. & Zheng, B. A reciprocal inhibition between ARID1 and MET1 in male and female gametes in Arabidopsis. J. Integr. Plant Biol. 59, 657–668 (2017).
Garcia-Aguilar, M., Michaud, C., Leblanc, O. & Grimanelli, D. Inactivation of a DNA methylation pathway in maize reproductive organs results in apomixis-like phenotypes. Plant Cell 22, 3249–3267 (2010).
Singh, M. et al. Production of viable gametes without meiosis in maize deficient for an ARGONAUTE protein. Plant Cell 23, 443–458 (2011).
Su, Z. et al. The THO complex non-cell-autonomously represses female germline specification through the TAS3-ARF3 module. Curr. Biol. 27, 1597–1609 e1592 (2017).
Su, Z. et al. Regulation of female germline specification via small RNA mobility in Arabidopsis. Plant Cell 32, 2842–2854 (2020).
Long, J. et al. Nurse cell–derived small RNAs define paternal epigenetic inheritance in Arabidopsis. Science 373, eabh0556 (2021).
Jiang, H. et al. MULTIPOLAR SPINDLE 1 (MPS1), a novel coiled-coil protein of Arabidopsis thaliana, is required for meiotic spindle organization. Plant J. 59, 1001–1010 (2009).
Wang, Z. et al. Polymerase IV plays a crucial role in pollen development in capsella. Plant Cell 32, 950–966 (2020).
Zhai, J. et al. Spatiotemporally dynamic, cell-type-dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl Acad. Sci. USA 112, 3146–3151 (2015).
Xia, R. et al. 24-nt reproductive phasiRNAs are broadly present in angiosperms. Nat. Commun. 10, 627 (2019).
Teng, C. et al. Dicer-like 5 deficiency confers temperature-sensitive male sterility in maize. Nat. Commun. 11, 2912 (2020).
Zhang, M. et al. CHH DNA methylation increases at 24-PHAS loci depend on 24-nt phased small interfering RNAs in maize meiotic anthers. New Phytol. 229, 2984–2997 (2021).
Zhou, X. et al. 24-nt phasiRNAs move from tapetal to meiotic cells in maize anthers. New Phytol. 235, 488–501 (2022).
Rodrigues, J. A. et al. Imprinted expression of genes and small RNA is associated with localized hypomethylation of the maternal genome in rice endosperm. Proc. Natl Acad. Sci. USA 110, 7934–7939 (2013).
Burgess, D., Chow, H. T., Grover, J. W., Freeling, M. & Mosher, R. A. Ovule siRNAs methylate protein-coding genes in trans. Plant Cell 34, 3647–3664 (2022).
Grover, J. W. et al. Abundant expression of maternal siRNAs is a conserved feature of seed development. Proc. Natl Acad. Sci. USA 117, 15305–15315 (2020).
Slotkin, R. K. et al. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461–472 (2009).
Calarco, J. P. et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 151, 194–205 (2012).
Ibarra, C. A. et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337, 1360–1364 (2012).
He, S., Vickers, M., Zhang, J. & Feng, X. Natural depletion of histone H1 in sex cells causes DNA demethylation, heterochromatin decondensation and transposon activation. eLife 8, e42530 (2019).
Kim, M. Y. et al. DNA demethylation by ROS1a in rice vegetative cells promotes methylation in sperm. Proc. Natl Acad. Sci. USA 116, 9652–9657 (2019).
Oliver, C. et al. The miRNome function transitions from regulating developmental genes to transposable elements during pollen maturation. Plant Cell 34, 784–801 (2022).
Martinez, G., Panda, K., Kohler, C. & Slotkin, R. K. Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nat. Plants 2, 16030 (2016).
Schroder, J. A., Bonnet, D. M. V. & Jullien, P. E. Non-cell-autonomous small RNA silencing in Arabidopsis female gametes. Curr. Biol. 33, 183–188 e183 (2023).
Erdmann, R. M. et al. Molecular movement in the Arabidopsis thaliana female gametophyte. Plant Reprod. 30, 141–146 (2017).
Borges, F. et al. Transposon-derived small RNAs triggered by miR845 mediate genome dosage response in Arabidopsis. Nat. Genet. 50, 186–192 (2018).
Papareddy, R. K. et al. Chromatin regulates expression of small RNAs to help maintain transposon methylome homeostasis in Arabidopsis. Genome Biol. 21, 251 (2020).
Zhou, S. et al. DNA demethylases remodel DNA methylation in rice gametes and zygote and are required for reproduction. Mol. Plant 14, 1569–1583 (2021).
Liu, Q. et al. Paternal DNA methylation is remodeled to maternal levels in rice zygote. Nat. Commun. 14, 6571 (2023).
Li, C. et al. Genome-wide redistribution of 24-nt siRNAs in rice gametes. Genome Res. 30, 173–184 (2020).
Li, C. et al. Resetting of the 24-nt siRNA landscape in rice zygotes. Genome Res. 32, 309–323 (2022).
Anderson, S. N. et al. The zygotic transition is initiated in unicellular plant zygotes with asymmetric activation of parental genomes. Dev. Cell 43, 349–358 e344 (2017).
Kawakatsu, T., Nery, J. R., Castanon, R. & Ecker, J. R. Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 18, 171 (2017).
Bouyer, D. et al. DNA methylation dynamics during early plant life. Genome Biol. 18, 179 (2017).
Kohler, C., Dziasek, K. & Del Toro-De Leon, G. Postzygotic reproductive isolation established in the endosperm: mechanisms, drivers and relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20200118 (2021).
Picard, C. L. & Gehring, M. Identification and comparison of imprinted genes across plant species. Methods Mol. Biol. 2093, 173–201 (2020).
Park, K. et al. DNA demethylation is initiated in the central cells of Arabidopsis and rice. Proc. Natl Acad. Sci. USA 113, 15138–15143 (2016).
Borg, M. et al. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol. 22, 621–629 (2020).
Luo, M., Bilodeau, P., Dennis, E. S., Peacock, W. J. & Chaudhury, A. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc. Natl Acad. Sci. USA 97, 10637–10642 (2000).
Pignatta, D. et al. Natural epigenetic polymorphisms lead to intraspecific variation in Arabidopsis gene imprinting. eLife 3, e03198 (2014).
Pignatta, D., Novitzky, K., Satyaki, P. R. V. & Gehring, M. A variably imprinted epiallele impacts seed development. PLoS Genet. 14, e1007469 (2018).
Moreno-Romero, J., Del Toro-De Leon, G., Yadav, V. K., Santos-Gonzalez, J. & Kohler, C. Epigenetic signatures associated with imprinted paternally expressed genes in the Arabidopsis endosperm. Genome Biol. 20, 41 (2019).
Kradolfer, D., Wolff, P., Jiang, H., Siretskiy, A. & Kohler, C. An imprinted gene underlies postzygotic reproductive isolation in Arabidopsis thaliana. Dev. Cell 26, 525–535 (2013).
Wolff, P., Jiang, H., Wang, G., Santos-Gonzalez, J. & Kohler, C. Paternally expressed imprinted genes establish postzygotic hybridization barriers in Arabidopsis thaliana. eLife 4, e10074 (2015).
Huang, F. et al. Mutants in the imprinted PICKLE RELATED 2 gene suppress seed abortion of fertilization independent seed class mutants and paternal excess interploidy crosses in Arabidopsis. Plant J. 90, 383–395 (2017).
Schatlowski, N. et al. Hypomethylated pollen bypasses the interploidy hybridization barrier in Arabidopsis. Plant Cell 26, 3556–3568 (2014).
Erdmann, R. M., Satyaki, P. R. V., Klosinska, M. & Gehring, M. A small RNA pathway mediates allelic dosage in endosperm. Cell Rep. 21, 3364–3372 (2017).
Jiang, H. et al. Ectopic application of the repressive histone modification H3K9me2 establishes post-zygotic reproductive isolation in Arabidopsis thaliana. Genes Dev. 31, 1272–1287 (2017).
Wang, Z. et al. Transgenerational effect of mutants in the RNA-directed DNA methylation pathway on the triploid block in Arabidopsis. Genome Biol. 22, 141 (2021).
Huc, J. et al. Bypassing reproductive barriers in hybrid seeds using chemically induced epimutagenesis. Plant Cell 34, 989–1001 (2022).
Liu, P. et al. DNA cytosine methylation dynamics and functional roles in horticultural crops. Hortic. Res. 10, uhad170 (2023).
Zhong, S. et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 31, 154–159 (2013).
Liu, R. et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl Acad. Sci. USA 112, 10804–10809 (2015).
Cheng, J. et al. Downregulation of RdDM during strawberry fruit ripening. Genome Biol. 19, 212 (2018).
Huang, H. et al. Global increase in DNA methylation during orange fruit development and ripening. Proc. Natl Acad. Sci. USA 116, 1430–1436 (2019).
Lu, P. et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants 4, 784–791 (2018).
Hollick, J. B. Paramutation and related phenomena in diverse species. Nat. Rev. Genet. 18, 5–23 (2017).
Cao, S. & Chen, Z. J. Transgenerational epigenetic inheritance during plant evolution and breeding. Trends Plant Sci., https://doi.org/10.1016/j.tplants.2024.04.007 (2024).
Cubas, P., Vincent, C. & Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401, 157–161 (1999).
Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549 (2010).
Manning, K. et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 38, 948–952 (2006).
Song, Q., Zhang, T., Stelly, D. M. & Chen, Z. J. Epigenomic and functional analyses reveal roles of epialleles in the loss of photoperiod sensitivity during domestication of allotetraploid cottons. Genome Biol. 18, 99 (2017).
Pilu, R. et al. A paramutation phenomenon is involved in the genetics of maize low phytic acid1-241 (lpa1-241) trait. Heredity 102, 236–245 (2009).
Kawanabe, T. et al. Role of DNA methylation in hybrid vigor in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 113, E6704–E6711 (2016).
Zhang, Q. et al. The chromatin remodeler DDM1 promotes hybrid vigor by regulating salicylic acid metabolism. Cell Discov. 2, 16027 (2016).
Brachi, B., Morris, G. P. & Borevitz, J. O. Genome-wide association studies in plants: the missing heritability is in the field. Genome Biol. 12, 232 (2011).
Kawakatsu, T. et al. Epigenomic diversity in a global collection of Arabidopsis thaliana accessions. Cell 166, 492–505 (2016).
Shirai, K. et al. Positive selective sweeps of epigenetic mutations regulating specialized metabolites in plants. Genome Res. 31, 1060–1068 (2021).
Eichten, S. R. et al. Epigenetic and genetic influences on DNA methylation variation in maize populations. Plant Cell 25, 2783–2797 (2013).
Xu, G. et al. Evolutionary and functional genomics of DNA methylation in maize domestication and improvement. Nat. Commun. 11, 5539 (2020).
Shen, Y. et al. DNA methylation footprints during soybean domestication and improvement. Genome Biol. 19, 128 (2018).
Zhao, L. et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 11, 2658 (2020).
Gardiner, L. J. et al. Hidden variation in polyploid wheat drives local adaptation. Genome Res. 28, 1319–1332 (2018).
Xu, J. et al. Population-level analysis reveals the widespread occurrence and phenotypic consequence of DNA methylation variation not tagged by genetic variation in maize. Genome Biol. 20, 243 (2019).
Schmitz, R. J. et al. Patterns of population epigenomic diversity. Nature 495, 193–198 (2013).
Dubin, M. J. et al. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. eLife 4, e05255 (2015).
Gouil, Q. & Baulcombe, D. C. Paramutation-like features of multiple natural epialleles in tomato. BMC Genomics 19, 203 (2018).
Cao, S. et al. Small RNAs mediate transgenerational inheritance of genome-wide trans-acting epialleles in maize. Genome Biol. 23, 53 (2022).
Cortijo, S. et al. Mapping the epigenetic basis of complex traits. Science 343, 1145–1148 (2014).
Zhang, Y. Y., Latzel, V., Fischer, M. & Bossdorf, O. Understanding the evolutionary potential of epigenetic variation: a comparison of heritable phenotypic variation in epiRILs, RILs, and natural ecotypes of Arabidopsis thaliana. Heredity 121, 257–265 (2018).
Schmidt, M. et al. Methylome and epialleles in rice epilines selected for energy use efficiency. Agronomy 8, 163 (2018).
Hauben, M. et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc. Natl Acad. Sci. USA 106, 20109–20114 (2009).
Huang, Y. et al. Genome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res. 8, 69 (2021).
Wu, R. et al. Inter-species grafting caused extensive and heritable alterations of DNA methylation in Solanaceae plants. PLoS ONE 8, e61995 (2013).
Johannes, F. & Schmitz, R. J. Spontaneous epimutations in plants. New Phytol. 221, 1253–1259 (2019).
Hazarika, R. R. et al. Molecular properties of epimutation hotspots. Nat. Plants 8, 146–156 (2022).
Blevins, T. et al. A two-step process for epigenetic inheritance in Arabidopsis. Mol. Cell 54, 30–42 (2014).
Li, J. et al. Epigenetic memory marks determine epiallele stability at loci targeted by de novo DNA methylation. Nat. Plants 6, 661–674 (2020).
Yang, X. et al. MutS HOMOLOG1-derived epigenetic breeding potential in tomato. Plant Physiol. 168, 222–232 (2015).
Raju, S. K. K. et al. An epigenetic breeding system in soybean for increased yield and stability. Plant Biotechnol. J. 16, 1836–1847 (2018).
Pan, C. et al. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 8, 513–525 (2022).
Gallego-Bartolome, J. et al. Targeted DNA demethylation of the Arabidopsis genome using the human TET1 catalytic domain. Proc. Natl Acad. Sci. USA 115, E2125–E2134 (2018).
Ji, L. et al. TET-mediated epimutagenesis of the Arabidopsis thaliana methylome. Nat. Commun. 9, 895 (2018).
Papikian, A., Liu, W., Gallego-Bartolome, J. & Jacobsen, S. E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 10, 729 (2019).
Ghoshal, B., Picard, C. L., Vong, B., Feng, S. & Jacobsen, S. E. CRISPR-based targeting of DNA methylation in Arabidopsis thaliana by a bacterial CG-specific DNA methyltransferase. Proc. Natl Acad. Sci. USA 118, e2125016118 (2021).
Galonska, C. et al. Genome-wide tracking of dCas9-methyltransferase footprints. Nat. Commun. 9, 597 (2018).
Pflueger, C. et al. A modular dCas9-SunTag DNMT3A epigenome editing system overcomes pervasive off-target activity of direct fusion dCas9-DNMT3A constructs. Genome Res. 28, 1193–1206 (2018).
Chan, W. F. et al. Activation of stably silenced genes by recruitment of a synthetic de-methylating module. Nat. Commun. 13, 5582 (2022).
Swain, T. et al. A modular dCas9-based recruitment platform for combinatorial epigenome editing. Nucleic Acids Res. 52, 474–491 (2024).
Li, Y. et al. An AP endonuclease functions in active DNA demethylation and gene imprinting in Arabidopsis [corrected]. PLoS Genet. 11, e1004905 (2015).
Li, J., Liang, W., Li, Y. & Qian, W. APURINIC/APYRIMIDINIC ENDONUCLEASE2 and ZINC FINGER DNA 3′-PHOSPHOESTERASE play overlapping roles in the maintenance of epigenome and genome stability. Plant. Cell 30, 1954–1970 (2018).
Li, Y., Duan, C. G., Zhu, X., Qian, W. & Zhu, J. K. A DNA ligase required for active DNA demethylation and genomic imprinting in Arabidopsis. Cell Res. 25, 757–760 (2015).
Martinez, G. et al. Paternal easiRNAs regulate parental genome dosage in Arabidopsis. Nat. Genet. 50, 193–198 (2018).
Sato, H., Santos-Gonzalez, J. & Kohler, C. Combinations of maternal-specific repressive epigenetic marks in the endosperm control seed dormancy. eLife 10, e64593 (2021).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR–Cas9 complex. Nature 517, 583–588 (2015).
Peabody, D. S. The RNA binding site of bacteriophage MS2 coat protein. EMBO J. 12, 595–600 (1993).
Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S. & Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Tucker, S. L., Reece, J., Ream, T. S. & Pikaard, C. S. Evolutionary history of plant multisubunit RNA polymerases IV and V: subunit origins via genome-wide and segmental gene duplications, retrotransposition, and lineage-specific subfunctionalization. Cold Spring Harb. Symp. Quant. Biol. 75, 285–297 (2010).
Ream, T. S. et al. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol. Cell 33, 192–203 (2009).
Acknowledgements
The authors apologize to all colleagues whose work could not be cited because of space limitations. They thank Y. Zhang from the CAS Center for Excellence in Molecular Plant Sciences for the careful reading and helpful discussion of the manuscript. This work was partly supported by the National Key Research and Development Program of China (2021YFA1300401 to H.Z. and 2021YFA1300400 to J.-K.Z.).
Author information
Authors and Affiliations
Contributions
J.-K.Z. made a substantial contribution to discussion of content, wrote and reviewed/edited the manuscript before submission. H.Z. researched data for the article, made a substantial contribution to discussion of content, wrote and reviewed/edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Ildoo Hwang, who co-reviewed with Jaemyung Choi, and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- De novo DNA methylation
-
A process of adding a methyl group to the cytosine base of unmethylated DNA. Among CG, CHG and CHH methylation, only CHH methylation is asymmetrical, that is, its complementary strand is not methylated. Thus, maintaining CHH methylation during DNA replication requires de novo methyltransferase activity.
- EasiRNAs
-
Secondary small interfering RNAs produced from AGO1-microRNA-cleaved transposable element transcripts. Production of easiRNAs in the pollen relies on DCL2, DCL4 and RNA polymerase IV.
- Epigenetic modification
-
Covalent modification of the DNA or histones that confers mitotically or meiotically heritable alterations of gene expression status but does not involve DNA sequence changes.
- Epigenetic recombination inbred lines
-
Epigenetic recombinant inbred lines are created by crosses between wild-type and hypomethylated (such as met1 or ddm1 mutant) plants followed by multiple generations of inbreeding.
- Gene body methylation
-
DNA methylation pattern associated with a subset of genes. It is characterized by medium to high levels of CG methylation and depletion of non-CG methylation in the gene body and low methylation around transcription start and termination sites.
- Interploidy hybridization barriers
-
Intraspecific hybridization barriers between individual plants with different ploidy levels, also known as the triploid block.
- Intragenic heterochromatin
-
A heterochromatin region located within an expressed protein-coding gene.
- Mutation accumulation lines
-
Plant lines that are propagated from a single founder through inbreeding and single-seed descent for several generations in the laboratory with the aim of minimizing the effect of natural selection and allowing the accumulation of spontaneous mutations (and epimutations).
- Paramutation
-
An epigenetic phenomenon whereby one allele induces heritable changes in the expression status of the other allele of the same locus. Paramutation has been discovered in both plants and animals.
- PhasiRNA
-
Secondary small interfering RNAs generated from the AGO-microRNA-cleaved mRNAs or long non-coding RNAs. The production of phasiRNAs involves RDR6, SGS3 and DCLs.
- Polymerase backtracking
-
Reversible sliding of a polymerase along the DNA or RNA template.
- Selective sweeps
-
Genomic regions with reduced genetic variation because a linked beneficial mutation increases in frequency and becomes fixed in the population.
- Siren siRNAs
-
A small number of highly expressed RNA polymerase IV-dependent small interfering RNAs (siRNAs) that were first reported in the rice endosperm. Siren siRNAs are also identified in female gametophytes and the surrounding sporophytic tissue.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Zhu, JK. Epigenetic gene regulation in plants and its potential applications in crop improvement. Nat Rev Mol Cell Biol (2024). https://doi.org/10.1038/s41580-024-00769-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41580-024-00769-1