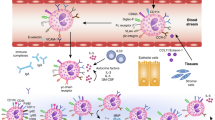
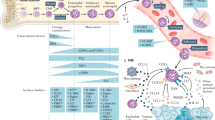
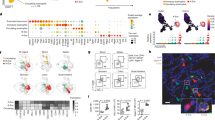
Abstract
Eosinophils are bone marrow-derived granulocytes that are traditionally associated with type 2 immune responses, such as those that occur during parasite infections and allergy. Emerging evidence demonstrates the remarkable functional plasticity of this elusive cell type and its pleiotropic functions in diverse settings. Eosinophils broadly contribute to tissue homeostasis, host defence and immune regulation, predominantly at mucosal sites. The scope of their activities primarily reflects the breadth of their portfolio of secreted mediators, which range from cytotoxic cationic proteins and reactive oxygen species to multiple cytokines, chemokines and lipid mediators. Here, we comprehensively review basic eosinophil biology that is directly related to their activities in homeostasis, protective immunity, regeneration and cancer. We examine how dysregulation of these functions contributes to the physiopathology of a broad range of inflammatory diseases. Furthermore, we discuss recent findings regarding the tissue compartmentalization and adaptation of eosinophils, shedding light on the factors that likely drive their functional diversification within tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rothenberg, M. E. & Hogan, S. P. The eosinophil. Annu. Rev. Immunol. 24, 147–174 (2006).
Mack, E. A. & Pear, W. S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr. Opin. Hematol. 27, 27–33 (2020).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 (2013).
Mishra, A., Hogan, S. P., Lee, J. J., Foster, P. S. & Rothenberg, M. E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Invest. 103, 1719–1727 (1999).
Xenakis, J. J. et al. Resident intestinal eosinophils constitutively express antigen presentation markers and include two phenotypically distinct subsets of eosinophils. Immunology 154, 298–308 (2018).
Foster, P. S. et al. Elemental signals regulating eosinophil accumulation in the lung. Immunol. Rev. 179, 173–181 (2001).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Gigon, L., Fettrelet, T., Yousefi, S., Simon, D. & Simon, H. U. Eosinophils from A to Z. Allergy 78, 1810–1846 (2023).
Yang, M. et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J. Allergy Clin. Immunol. 112, 935–943 (2003).
Ben Baruch-Morgenstern, N. et al. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat. Immunol. 15, 36–44 (2014).
Moshkovits, I. et al. CMRF35-like molecule 1 (CLM-1) regulates eosinophil homeostasis by suppressing cellular chemotaxis. Mucosal Immunol. 7, 292–303 (2014).
Verjan Garcia, N. et al. SIRPalpha/CD172a regulates eosinophil homeostasis. J. Immunol. 187, 2268–2277 (2011).
Munitz, A. et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 107, 1996–2003 (2006).
Gurtner, A. et al. Active eosinophils regulate host defence and immune responses in colitis. Nature 615, 151–157 (2023). The first comprehensive single-cell RNA sequencing analysis of eosinophils, which describes the spatial and tissue-specific adaptation of GI eosinophils.
Gurtner, A., Crepaz, D. & Arnold, I. A. Emerging functions of tissue-resident eosinophils. J. Exp. Med. 220, e20221435 (2023).
Wen, T. et al. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J. Immunol. 188, 1075–1082 (2012).
Diny, N. L. et al. The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice. J. Exp. Med. 219, e20210970 (2022).
Li, Y. et al. Neuromedin U programs eosinophils to promote mucosal immunity of the small intestine. Science 381, 1189–1196 (2023). This study demonstrates that neuromedin U has a crucial role in controlling epithelial cell differentiation and barrier immunity through the activation of NMUR1-expresssing eosinophills in the small intestine, emphasizing the significance of neuroimmune–epithelial communication in sustaining tissue equilibrium.
Larsen, L. D., Dockstader, K., Olbrich, C. L., Cartwright, I. M. & Spencer, L. A. Modulation of surface CD11c expression tracks plasticity in murine intestinal tissue eosinophils. J. Leukoc. Biol. 111, 943–952 (2022).
Kutyavin, V. I., Korn, L. L. & Medzhitov, R. Nutrient-derived signals regulate eosinophil adaptation to the small intestine. Proc. Natl Acad. Sci. USA 121, e2316446121 (2024).
Munitz, A. et al. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J. Allergy Clin. Immunol. 122, 1200–1207.e1 (2008).
Schanz, O. et al. Dietary AhR ligands regulate AhRR expression in intestinal immune cells and intestinal microbiota composition. Int. J. Mol. Sci. 21, 3189 (2020).
Lee, J. J., Jacobsen, E. A., McGarry, M. P., Schleimer, R. P. & Lee, N. A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575 (2010).
Jacobsen, E. A. et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu. Rev. Immunol. 39, 719–757 (2021).
Jackson, D. J. & Pavord, I. D. Living without eosinophils: evidence from mouse and man. Eur. Respir. J. 61, 2201217 (2023).
Ignacio, A. et al. Small intestinal resident eosinophils maintain gut homeostasis following microbial colonization. Immunity 55, 1250–1267.e12 (2022).
Chu, V. T. et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40, 582–593 (2014).
Jung, Y. et al. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 8, 930–942 (2015).
Sturm, N. et al. Spatial heterogeneity for APRIL production by eosinophils in the small intestine. J. Leukoc. Biol. 113, 376–382 (2023).
Sugawara, R. et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J. Exp. Med. 213, 555–567 (2016).
Fallegger, A. et al. TGF-β production by eosinophils drives the expansion of peripherally induced neuropilin− RORγ+ regulatory T-cells during bacterial and allergen challenge. Mucosal Immunol. 15, 504–514 (2022).
McGuire, J. K., Li, Q. & Parks, W. C. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am. J. Pathol. 162, 1831–1843 (2003).
FitzPatrick, R. D. et al. Littermate-controlled experiments reveal eosinophils are not essential for maintaining steady-state IgA and demonstrate the influence of rearing conditions on antibody phenotypes in eosinophil-deficient mice. Front. Immunol. 11, 557960 (2020).
Loffredo, L. F. et al. Eosinophil accumulation in postnatal lung is specific to the primary septation phase of development. Sci. Rep. 10, 4425 (2020).
Saluzzo, S. et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep. 18, 1893–1905 (2017).
Mesnil, C. et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Invest. 126, 3279–3295 (2016).
Chojnacki, A. et al. Intravital imaging allows real-time characterization of tissue resident eosinophils. Commun. Biol. 2, 181 (2019).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Zhu, C. et al. Homeostatic and early-recruited CD101− eosinophils suppress endotoxin-induced acute lung injury. Eur. Respir. J. 56,1902354 (2020).
Masterson, J. C. et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 64, 1236–1247 (2015).
Dolitzky, A. et al. Mouse resident lung eosinophils are dependent on IL-5. Allergy 77, 2822–2825 (2022).
Throsby, M., Herbelin, A., Pleau, J. M. & Dardenne, M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J. Immunol. 165, 1965–1975 (2000).
Gatti, D. M. et al. MHCII+CD80+ thymic eosinophils increase in abundance during neonatal development in mice and their accumulation is microbiota dependent. J. Leukoc. Biol. 114, 223–236 (2023).
Odemuyiwa, S. O. et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J. Immunol. 173, 5909–5913 (2004).
Muller, E. Localization of eosinophils in the thymus by the peroxidase reaction. Histochemistry 52, 273–279 (1977).
Rytomaa, T. Organ distribution and histochemical properties of eosinophil granulocytes in rat. Acta Pathol. Microbiol. Scand. Suppl. 50 (Suppl. 140), 1–118 (1960).
Ross, R. & Klebanoff, S. J. The eosinophilic leukocyte. Fine structure studies of changes in the uterus during the estrous cycle. J. Exp. Med. 124, 653–660 (1966).
Cosway, E. J. et al. Eosinophils are an essential element of a type 2 immune axis that controls thymus regeneration. Sci. Immunol. 7, eabn3286 (2022).
Dolitzky, A. et al. Differential regulation of type 1 and type 2 mouse eosinophil activation by apoptotic cells. Front. Immunol. 13, 1041660 (2022).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Huang, Z. et al. The FGF21–CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 26, 493–508.e4 (2017).
Rana, B. M. J. et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 216, 1999–2009 (2019).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Lee, E. H. et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci. Rep. 8, 9894 (2018).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Bolus, W. R. et al. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol. Metab. 8, 86–95 (2018).
Dolitzky, A. et al. Transcriptional profiling of mouse eosinophils identifies distinct gene signatures following cellular activation. Front. Immunol. 12, 802839 (2021).
Shim, W. S. et al. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res. Clin. Pract. 73, 284–291 (2006).
Sunadome, H. et al. Correlation between eosinophil count, its genetic background and body mass index: the Nagahama study. Allergol. Int. 69, 46–52 (2020).
Hashiguchi, M. et al. IL-33 activates eosinophils of visceral adipose tissue both directly and via innate lymphoid cells. Eur. J. Immunol. 45, 876–885 (2015).
Brychta, R. J. & Chen, K. Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 71, 345–352 (2017).
Bang, I. H. et al. Sirtuin 6 promotes eosinophil differentiation by activating GATA-1 transcription factor. Aging Cell 20, e13418 (2021).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Fischer, K. et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23, 623–630 (2017).
Withers, S. B. et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci. Rep. 7, 44571 (2017).
Lee, M. W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Michalopoulos, G. K. & Bhushan, B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 18, 40–55 (2021).
Goh, Y. P. et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl Acad. Sci. USA 110, 9914–9919 (2013).
Xu, L. et al. Hepatic recruitment of eosinophils and their protective function during acute liver injury. J. Hepatol. 77, 344–352 (2022).
Wang, Y. et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci. Transl Med. 13, eabb6576 (2021).
Hart, K. M. et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci. Transl Med. 9, eaal3694 (2017).
Sciorati, C., Rigamonti, E., Manfredi, A. A. & Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 23, 927–937 (2016).
Heredia, J. E. et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013).
Huang, L. et al. Eosinophils and IL-4 support nematode growth coincident with an innate response to tissue injury. PLoS Pathog. 11, e1005347 (2015).
Kastenschmidt, J. M. et al. A stromal progenitor and ILC2 niche promotes muscle eosinophilia and fibrosis-associated gene expression. Cell Rep. 35, 108997 (2021).
Sek, A. C. et al. Eosinophils do not drive acute muscle pathology in the mdx mouse model of duchenne muscular dystrophy. J. Immunol. 203, 476–484 (2019).
Pan, D. et al. IL-4 expressing cells are recruited to nerve after injury and promote regeneration. Exp. Neurol. 347, 113909 (2022).
Liebendorfer, A. et al. Loss of Gata1 decreased eosinophils, macrophages, and type 2 cytokines in regenerating nerve and delayed axon regeneration after a segmental nerve injury. Exp. Neurol. 362, 114327 (2023).
Mitre, E. & Klion, A. D. Eosinophils and helminth infection: protective or pathogenic. Semin. Immunopathol. 43, 363–381 (2021).
Gaur, P. et al. The regulatory role of eosinophils in viral, bacterial, and fungal infections. Clin. Exp. Immunol. 209, 72–82 (2022).
Yousefi, S. et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14, 949–953 (2008). This study demonstrates a previously undescribed mechanism of eosinophil-mediated innate immune responses via the release of mitochondrial DNA in a catapult-like manner.
Cheung, P. F., Wong, C. K., Ip, W. K. & Lam, C. W. FAK-mediated activation of ERK for eosinophil migration: a novel mechanism for infection-induced allergic inflammation. Int. Immunol. 20, 353–363 (2008).
Driss, V. et al. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood 113, 3235–3244 (2009).
Plotz, S. G. et al. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood 97, 235–241 (2001).
Arnold, I. C. et al. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 215, 2055–2072 (2018). This study demonstrates that intestinal eosinophils regulate tissue homeostasis by suppressing TH1 cell responses and can display bactericidal activities towards Citrobacter.
Bohrer, A. C. et al. Eosinophils are part of the granulocyte response in tuberculosis and promote host resistance in mice. J. Exp. Med. 218, e20210469 (2021).
Bohrer, A. C. et al. Rapid GPR183-mediated recruitment of eosinophils to the lung after Mycobacterium tuberculosis infection. Cell Rep. 40, 111144 (2022).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Phipps, S. et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110, 1578–1586 (2007).
Yoon, J., Ponikau, J. U., Lawrence, C. B. & Kita, H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J. Immunol. 181, 2907–2915 (2008).
Matsuwaki, Y. et al. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J. Immunol. 183, 6708–6716 (2009).
Ishikawa, T., Yu, M. C. & Arbesman, C. E. Electron microscopic demonstration of phagocytosis of Candida albicans by human eosinophilic leukocytes. J. Allergy Clin. Immunol. 50, 183–187 (1972).
Ueki, S. et al. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 137, 258–267 (2016).
Lilly, L. M. et al. Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect. Immun. 82, 1315–1325 (2014).
Kobayashi, T., Kouzaki, H. & Kita, H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J. Immunol. 184, 6350–6358 (2010).
Lotfi, R. et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 183, 5023–5031 (2009).
Jans, J. et al. Fc gamma receptors in respiratory syncytial virus infections: implications for innate immunity. Rev. Med. Virol. 24, 55–70 (2014).
Erbe, D. V., Pfefferkorn, E. R. & Fanger, M. W. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J. Immunol. 146, 3145–3151 (1991).
Ikeda, Y., Mita, H., Kudo, M., Hasegawa, M. & Akiyama, K. Degranulation of eosinophils by IgG antibody to Candida antigen [Japanese]. Arerugi 48, 546–553 (1999).
Esnault, S. et al. Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling. Clin. Exp. Allergy 50, 198–212 (2020).
Bartemes, K. R., Cooper, K. M., Drain, K. L. & Kita, H. Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions. J. Allergy Clin. Immunol. 116, 827–835 (2005).
Pleass, R. J., Lang, M. L., Kerr, M. A. & Woof, J. M. IgA is a more potent inducer of NADPH oxidase activation and degranulation in blood eosinophils than IgE. Mol. Immunol. 44, 1401–1408 (2007).
Gounni, A. S. et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 367, 183–186 (1994).
Seltmann, J., Werfel, T. & Wittmann, M. Evidence for a regulatory loop between IFN-gamma and IL-33 in skin inflammation. Exp. Dermatol. 22, 102–107 (2013).
Dahlgren, M. W. et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722.e6 (2019).
Dwyer, G. K., D’Cruz, L. M. & Turnquist, H. R. Emerging functions of IL-33 in homeostasis and immunity. Annu. Rev. Immunol. 40, 15–43 (2022).
Bouffi, C. et al. IL-33 markedly activates murine eosinophils by an NF-κB-dependent mechanism differentially dependent upon an IL-4-driven autoinflammatory loop. J. Immunol. 191, 4317–4325 (2013).
Shik, D., Moshkovits, I., Karo-Atar, D., Reichman, H. & Munitz, A. Interleukin-33 requires CMRF35-like molecule-1 expression for induction of myeloid cell activation. Allergy 69, 719–729 (2014).
Zaph, C. et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198 (2008).
Buonomo, E. L. et al. Microbiota-regulated IL-25 increases eosinophil number to provide protection during Clostridium difficile infection. Cell Rep. 16, 432–443 (2016).
Tang, W. et al. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int. Arch. Allergy Immunol. 163, 5–10 (2014).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Hatano, Y. et al. Phagocytosis of heat-killed Staphylococcus aureus by eosinophils: comparison with neutrophils. APMIS 117, 115–123 (2009).
Lehrer, R. I. et al. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J. Immunol. 142, 4428–4434 (1989).
Melo, R. C. & Weller, P. F. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol. Histopathol. 25, 1341–1354 (2010).
Ueki, S. et al. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 121, 2074–2083 (2013).
Ueki, S. et al. Charcot–Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 132, 2183–2187 (2018).
Muniz, V. S. et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J. Allergy Clin. Immunol. 141, 571–585.e7 (2018).
Ehrens, A. et al. Microfilariae trigger eosinophil extracellular DNA traps in a dectin-1-dependent manner. Cell Rep. 34, 108621 (2021).
Gigon, L., Yousefi, S., Karaulov, A. & Simon, H. U. Mechanisms of toxicity mediated by neutrophil and eosinophil granule proteins. Allergol. Int. 70, 30–38 (2021).
Svensson, L. & Wenneras, C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 7, 720–728 (2005).
Khatun, A., Sakurai, M., Okada, K., Sakai, Y. & Morimoto, M. Detection of alpha-defensin in eosinophils in helminth-infected mouse model. J. Vet. Med. Sci. 80, 1887–1894 (2018).
Harris, T. A. et al. Resistin-like molecule alpha provides vitamin-A-dependent antimicrobial protection in the skin. Cell Host Microbe 25, 777–788.e8 (2019).
Cogan, E. & Roufosse, F. Clinical management of the hypereosinophilic syndromes. Expert Rev. Hematol. 5, 275–289 (2012).
Egan, M. & Furuta, G. T. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 121, 162–167 (2018).
O’Shea, K. M. et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology 154, 333–345 (2018).
Mishra, A. et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology 134, 204–214 (2008).
Rothenberg, M. E. et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat. Genet. 42, 289–291 (2010).
Sleiman, P. M. et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat. Commun. 5, 5593 (2014).
Blanchard, C. et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 120, 1292–1300 (2007).
Mueller, S., Aigner, T., Neureiter, D. & Stolte, M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J. Clin. Pathol. 59, 1175–1180 (2006).
Dunn, J. L. M. et al. Esophageal type 2 cytokine expression heterogeneity in eosinophilic esophagitis in a multisite cohort. J. Allergy Clin. Immunol. 145, 1629–1640.e4 (2020).
Mavi, P., Rajavelu, P., Rayapudi, M., Paul, R. J. & Mishra, A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1347–G1355 (2012).
Kliewer, K. L. et al. Benralizumab for eosinophilic gastritis: a single-site, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 803–815 (2023).
Tappata, M. et al. Association of mast cells with clinical, endoscopic, and histologic findings in adults with eosinophilic esophagitis. Allergy 73, 2088–2092 (2018).
Morgan, D. M. et al. Clonally expanded, GPR15-expressing pathogenic effector TH2 cells are associated with eosinophilic esophagitis. Sci. Immunol. 6, eabi5586 (2021).
Jeziorska, M., Haboubi, N., Schofield, P. & Woolley, D. E. Distribution and activation of eosinophils in inflammatory bowel disease using an improved immunohistochemical technique. J. Pathol. 194, 484–492 (2001).
Forbes, E. et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J. Immunol. 172, 5664–5675 (2004).
Loktionov, A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J. Gastroenterol. 25, 3503–3526 (2019).
Manousou, P. et al. Increased expression of chemokine receptor CCR3 and its ligands in ulcerative colitis: the role of colonic epithelial cells in in vitro studies. Clin. Exp. Immunol. 162, 337–347 (2010).
Ahrens, R. et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 181, 7390–7399 (2008).
Filippone, R. T. et al. Potent CCR3 receptor antagonist, SB328437, suppresses colonic eosinophil chemotaxis and inflammation in the winnie murine model of spontaneous chronic colitis. Int. J. Mol. Sci. 23, 7780 (2022).
Jacobs, I. et al. Role of eosinophils in intestinal inflammation and fibrosis in inflammatory bowel disease: an overlooked villain? Front. Immunol. 12, 754413 (2021).
Filippone, R. T., Sahakian, L., Apostolopoulos, V. & Nurgali, K. Eosinophils in inflammatory bowel disease. Inflamm. Bowel Dis. 25, 1140–1151 (2019).
Nishitani, H., Okabayashi, M., Satomi, M., Shimoyama, T. & Dohi, Y. Infiltration of peroxidase-producing eosinophils into the lamina propria of patients with ulcerative colitis. J. Gastroenterol. 33, 189–195 (1998).
Saitoh, O. et al. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am. J. Gastroenterol. 94, 3513–3520 (1999).
Griseri, T. et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43, 187–199 (2015).
Kobori, A. et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 45, 999–1007 (2010).
De Salvo, C. et al. IL-33 drives eosinophil infiltration and pathogenic type 2 helper T-cell immune responses leading to chronic experimental ileitis. Am. J. Pathol. 186, 885–898 (2016).
Masterson, J. C. et al. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn’s ileitis. Inflamm. Bowel Dis. 21, 2429–2440 (2015).
Villanacci, V. et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 20, 1009–1016 (2008).
Smyth, C. M. et al. Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS ONE 8, e64216 (2013).
Filippone, R. T. et al. Targeting eotaxin-1 and CCR3 receptor alleviates enteric neuropathy and colonic dysfunction in TNBS-induced colitis in guinea pigs. Neurogastroenterol. Motil. 30, e13391 (2018).
Lampinen, M. et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut 54, 1714–1720 (2005).
McBrien, C. N. & Menzies-Gow, A. The biology of eosinophils and their role in asthma. Front. Med. 4, 93 (2017).
Nakagome, K. & Nagata, M. Innate immune responses by respiratory viruses, including rhinovirus, during asthma exacerbation. Front. Immunol. 13, 865973 (2022).
Bousquet, J. et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 323, 1033–1039 (1990).
Price, D. B. et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir. Med. 3, 849–858 (2015).
Yancey, S. W. et al. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 140, 1509–1518 (2017).
Ortega, H. G. et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371, 1198–1207 (2014). A key study demonstrating that targeting eosinophils via neutralizing IL-5 (using mepolizumab) reduced asthma exacerbations and was associated with improvements in markers of asthma control.
FitzGerald, J. M. et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 388, 2128–2141 (2016).
Gundel, R. H., Letts, L. G. & Gleich, G. J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J. Clin. Invest. 87, 1470–1473 (1991).
Coyle, A. J., Ackerman, S. J., Burch, R., Proud, D. & Irvin, C. G. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J. Clin. Invest. 95, 1735–1740 (1995).
Piliponsky, A. M., Pickholtz, D., Gleich, G. J. & Levi-Schaffer, F. Human eosinophils induce histamine release from antigen-activated rat peritoneal mast cells: a possible role for mast cells in late-phase allergic reactions. J. Allergy Clin. Immunol. 107, 993–1000 (2001).
Grunig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).
Makinde, T., Murphy, R. F. & Agrawal, D. K. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol. Cell Biol. 85, 348–356 (2007).
Humbles, A. A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004). This study uses eosinophil-deficent ΔdblGATA mice to demonstrate that eosinophils contribute to tissue remodelling in asthma.
Lee, J. J. et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004). This study describes the generation of an additional eosinophil-deficient mouse line (PHIL mice), which was used to demonstrate the requirement of eosinophils for the development of pulmonary mucus accumulation and AHR associated with asthma.
Drake, M. G. et al. Eosinophil and airway nerve interactions in asthma. J. Leukoc. Biol. 104, 61–67 (2018).
Persson, E. K. et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 364, eaaw4295 (2019).
Nyenhuis, S. M. et al. Charcot–Leyden crystal protein/galectin-10 is a surrogate biomarker of eosinophilic airway inflammation in asthma. Biomark. Med. 13, 715–724 (2019).
Kaur, R. & Chupp, G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J. Allergy Clin. Immunol. 144, 1–12 (2019).
Flood-Page, P. et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am. J. Respir. Crit. Care Med. 176, 1062–1071 (2007).
Bafadhel, M. et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 184, 662–671 (2011).
Pavord, I. D. et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med. 377, 1613–1629 (2017).
Criner, G. J. et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir. Med. 8, 158–170 (2020).
Bafadhel, M. et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 186, 48–55 (2012).
Eltboli, O. et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm. Med. 14, 112 (2014).
Tworek, D. et al. The association between airway eosinophilic inflammation and IL-33 in stable non-atopic COPD. Respir. Res. 19, 108 (2018).
Jacobsen, E. A. et al. Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice. Allergy 70, 1148–1159 (2015).
Doyle, A. D. et al. Eosinophil-derived IL-13 promotes emphysema. Eur. Respir. J. 53, 1801291 (2019).
Calus, L. et al. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin. Transl. Allergy 9, 30 (2019).
Simon, H. U. et al. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J. Immunol. 158, 3902–3908 (1997).
Yun, Y. et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 69, 232–238 (2020).
Hauser, L. J., Chandra, R. K., Li, P. & Turner, J. H. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int. Forum Allergy Rhinol. 7, 957–962 (2017).
Saitoh, T. et al. Relationship between epithelial damage or basement membrane thickness and eosinophilic infiltration in nasal polyps with chronic rhinosinusitis. Rhinology 47, 275–279 (2009).
Howarth, P. et al. Severe eosinophilic asthma with nasal polyposis: a phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J. Allergy Clin. Immunol. 145, 1713–1715 (2020).
Han, J. K. et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 9, 1141–1153 (2021).
Stevens, W. W. et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am. J. Respir. Crit. Care Med. 192, 682–694 (2015).
Gevaert, E. et al. Charcot–Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J. Allergy Clin. Immunol. 145, 427–430.e4 (2020).
Kiehl, P., Falkenberg, K., Vogelbruch, M. & Kapp, A. Tissue eosinophilia in acute and chronic atopic dermatitis: a morphometric approach using quantitative image analysis of immunostaining. Br. J. Dermatol. 145, 720–729 (2001).
Cheng, J. F. et al. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J. Allergy Clin. Immunol. 99, 683–692 (1997).
Morshed, M., Yousefi, S., Stockle, C., Simon, H. U. & Simon, D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy 67, 1127–1137 (2012).
Simon, D. et al. Eosinophil extracellular DNA traps in skin diseases. J. Allergy Clin. Immunol. 127, 194–199 (2011).
Nakashima, C., Ishida, Y., Kitoh, A., Otsuka, A. & Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 28, 1405–1411 (2019).
Ruzicka, T. & Mihara, R. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 376, 2093 (2017).
Lee, J. J. et al. Eosinophil-dependent skin innervation and itching following contact toxicant exposure in mice. J. Allergy Clin. Immunol. 135, 477–487 (2015).
Kang, E. G. et al. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy 75, 950–953 (2020).
Kimura, R., Sugita, K., Horie, T. & Yamamoto, O. Dual role of basophils in the pathogenesis of bullous pemphigoid elucidated by pathological and ultrastructural studies. Eur. J. Dermatol. 32, 322–333 (2022).
Limberg, M. M. et al. Eosinophils, basophils, and neutrophils in bullous pemphigoid. Biomolecules 13, 1019 (2023).
de Graauw, E. et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy 72, 1105–1113 (2017).
Amber, K. T., Chernyavsky, A., Agnoletti, A. F., Cozzani, E. & Grando, S. A. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp. Dermatol. 27, 1322–1327 (2018).
Rudrich, U. et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm. Venereol. 98, 766–771 (2018).
Grisaru-Tal, S. et al. Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations. Oncoimmunology 10, 1859732 (2020).
Grisaru-Tal, S., Itan, M., Klion, A. D. & Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 20, 594–607 (2020).
Munitz, A. et al. 2B4 (CD244) is expressed and functional on human eosinophils. J. Immunol. 174, 110–118 (2005).
Kataoka, S., Konishi, Y., Nishio, Y., Fujikawa-Adachi, K. & Tominaga, A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 23, 549–560 (2004).
Simson, L. et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J. Immunol. 178, 4222–4229 (2007).
Lucarini, V. et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 6, e1317420 (2017).
Gatault, S. et al. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J. Immunol. 195, 2483–2492 (2015).
Legrand, F. et al. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 185, 7443–7451 (2010).
Andreone, S. et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 11, 1664 (2019).
Reichman, H. et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol. Res. 7, 388–400 (2019).
Mattes, J. et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Ikutani, M. et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 188, 703–713 (2012).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Grisaru-Tal, S. et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. 81, 5555–5571 (2021). This study demonstrates that the TME in lung metastasis shapes eosinophils to acquire lymphocyte-dependent antitumorigenic activities.
Carretero, R. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 16, 609–617 (2015).
Arnold, I. C. et al. The GM-CSF–IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. J. Exp. Med. 217, e20190706 (2020).
Onyema, O. O. et al. Eosinophils downregulate lung alloimmunity by decreasing TCR signal transduction. JCI Insight 4, e128241 (2019).
Zaynagetdinov, R. et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 75, 1624–1634 (2015).
Li, F. et al. Eosinophilic inflammation promotes CCL6-dependent metastatic tumor growth. Sci. Adv. 7, eabb5943 (2021).
Grisaru-Tal, S., Rothenberg, M. E. & Munitz, A. Eosinophil–lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat. Immunol. 23, 1309–1316 (2022).
Zheng, X. et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 146, 1730–1740 (2020).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Blomberg, O. S. et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 41, 106–123.e10 (2023). This study demonstrates that eosinophils contribute to the beneficial response after treatment with ICB by actively recruiting cytotoxic T cells into the tumour.
Jia, Q. et al. Peripheral eosinophil counts predict efficacy of anti-CD19 CAR-T cell therapy against B-lineage non-Hodgkin lymphoma. Theranostics 11, 4699–4709 (2021).
Cheng, J. N. et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci. Adv. 7, eabc7609 (2021).
Lai, W. et al. Human pluripotent stem cell-derived eosinophils reveal potent cytotoxicity against solid tumors. Stem Cell Rep. 16, 1697–1704 (2021).
Hollande, C. et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 20, 257–264 (2019).
Auciello, F. R. et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 9, 617–627 (2019).
Bhattacharyya, S. et al. Autotaxin-lysolipid signaling suppresses a CCL11-eosinophil axis to promote pancreatic cancer progression. Nat. Cancer 5, 283–298 (2024).
Fulkerson, P. C. Transcription factors in eosinophil development and as therapeutic targets. Front. Med. 4, 115 (2017).
Iwasaki, H. et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 201, 1891–1897 (2005).
Mori, Y. et al. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 206, 183–193 (2009).
Querfurth, E. et al. Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors. Genes Dev. 14, 2515–2525 (2000).
Bettigole, S. E. et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 16, 829–837 (2015).
Lu, T. X. et al. MiR-223 deficiency increases eosinophil progenitor proliferation. J. Immunol. 190, 1576–1582 (2013).
Lu, T. X. et al. Targeted ablation of miR-21 decreases murine eosinophil progenitor cell growth. PLoS ONE 8, e59397 (2013).
Wagner, L. A. et al. EGO, a novel, noncoding RNA gene, regulates eosinophil granule protein transcript expression. Blood 109, 5191–5198 (2007).
Sanderson, C. J. Interleukin-5, eosinophils, and disease. Blood 79, 3101–3109 (1992).
Collins, P. D., Marleau, S., Griffiths Johnson, D. A., Jose, P. J. & Williams, T. J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182, 1169–1174 (1995).
Rothenberg, M. E. et al. IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J. Immunol. 143, 2311–2316 (1989).
Johnston, L. K. et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J. Immunol. 197, 3445–3453 (2016).
Martens, A. et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res. 22, 2908–2918 (2016).
Lang, B. M. et al. Long-term survival with modern therapeutic agents against metastatic melanoma—vemurafenib and ipilimumab in a daily life setting. Med. Oncol. 35, 24 (2018).
Simon, S. C. S. et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 9, 1727116 (2020).
Acknowledgements
This work was supported by an Eccellenza Professorial Fellowship (PCEFP3_187021) and a Consolidator Grant (TMCG-3_213857) from the Swiss National Science Foundation to I.C.A., the US–Israel Bi-national Science Foundation (grant no. 2015163), Israel Science Foundation (grants no. 542/20), Israel Cancer Research Fund, Israel Cancer Association, Dotan Hemato-oncology Fund, Cancer Biology Research Center, Tel Aviv University and Azrieli Foundation Canada-Israel to A.M.
Author information
Authors and Affiliations
Contributions
I.C.A. and A.M conceived and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Lisa Spencer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- ΔdblGATA mice
-
Genetically modified mice that have been engineered to lack both copies of GATA1 in their genome, resulting in complete ablation of the eosinophil lineage.
- Alarmins
-
A termed used for a group of endogenous proteins or peptides with chemotactic and immune cell-activating properties that are released upon cell injury or death.
- Alanine transaminase
-
(ALT). An enzyme mainly expressed in the liver, but released into the blood following liver cell damage. An ALT test measures the amount of ALT in the blood.
- Alternatively activated macrophage
-
A term used to describe macrophages activated in vitro in an anti-inflammatory manner with IL-4 or IL-10. In vivo macrophages are highly specialized, transcriptomically dynamic and extremely heterogeneous with regard to their phenotypes and functions, which are continuously shaped by their tissue microenvironment.
- Central tolerance
-
A crucial process in the immune system that occurs in the thymus where developing T cells that recognize self-antigens with high affinity undergo apoptotic cell death or are rendered functionally inactive.
- Fibro-adipogenic progenitors
-
(FAPs). A type of mesenchymal stem cell found within skeletal muscle tissues that have significant roles in the context of muscle regeneration by differentiating into myofibroblasts and subsequently contributing to the generation of new muscle fibres.
- Fractional exhaled nitric oxide
-
(FeNO). A noninvasive biomarker that is used to assess airway inflammation, particularly in diseases such as asthma. It measures the concentration of nitric oxide gas in a person’s exhaled breath.
- Pruritus
-
A medical term used to describe the sensation that prompts a desire to scratch an affected area of the skin.
- Side-scatter
-
(SSC). A term used in flow cytometry to describe a measurement that can be used to measure the granularity or internal complexity of a cell.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arnold, I.C., Munitz, A. Spatial adaptation of eosinophils and their emerging roles in homeostasis, infection and disease. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01048-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01048-y