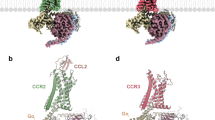
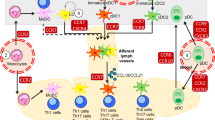
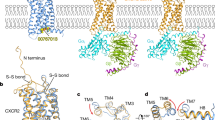
Abstract
Leukocyte migration is a fundamental component of innate and adaptive immune responses as it governs the recruitment and localization of these motile cells, which is crucial for immune cell priming, effector functions, memory responses and immune regulation. This complex cellular trafficking system is controlled to a large extent via highly regulated production of secreted chemokines and the restricted expression of their membrane-tethered G-protein-coupled receptors. The activity of chemokines and their receptors is also regulated by a subfamily of molecules known as atypical chemokine receptors (ACKRs), which are chemokine receptor-like molecules that do not couple to the classical signalling pathways that promote cell migration in response to chemokine ligation. There has been a great deal of progress in understanding the biology of these receptors and their functions in the immune system in the past decade. Here, we describe the contribution of the various ACKRs to innate and adaptive immune responses, focussing specifically on recent progress. This includes recent findings that have defined the role for ACKRs in sculpting extracellular chemokine gradients, findings that broaden the spectrum of chemokine ligands recognized by these receptors, candidate new additions to ACKR family, and our increasing understanding of the role of these receptors in shaping the migration of innate and adaptive immune cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bachelerie, F. et al. New nomenclature for atypical chemokine receptors. Nat. Immunol. 15, 207–208 (2014).
Nibbs, R. J. B. & Graham, G. J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13, 815–829 (2013).
Melgrati, S. et al. Atlas of the anatomical localization of atypical chemokine receptors in healthy mice. PLoS Biol. 21, e3002111 (2023). This study used novel GPR182 reporter mice and other existing ACKR reporter mice and chimeric tagged chemokines to comprehensively identify the distribution pattern of ACKRs throughout the body.
Rajagopal, S. et al. β-Arrestin- but not G protein-mediated signaling by the ‘decoy’ receptor CXCR7. Proc. Natl Acad. Sci. USA 107, 628–632 (2010).
Hoffmann, F. et al. Rapid uptake and degradation of CXCL12 depend on CXCR7 carboxyl-terminal serine/threonine residues. J. Biol. Chem. 287, 28362–28377 (2012).
Galliera, E. et al. beta-Arrestin-dependent constitutive internalization of the human chemokine decoy receptor D6. J. Biol. Chem. 279, 25590–25597 (2004).
Watts, A. O. et al. β-Arrestin recruitment and G protein signaling by the atypical human chemokine decoy receptor CCX-CKR. J. Biol. Chem. 288, 7169–7181 (2013).
Nguyen, H. T. et al. CXCR7: a β-arrestin-biased receptor that potentiates cell migration and recruits β-arrestin2 exclusively through Gβγ subunits and GRK2. Cell Biosci. 10, 134 (2020).
Weber, M. et al. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell 15, 2492–2508 (2004).
McCulloch, C. V. et al. Multiple roles for the C-terminal tail of the chemokine scavenger D6. J. Biol. Chem. 283, 7972–7982 (2008).
Canals, M. et al. Ubiquitination of CXCR7 controls receptor trafficking. PLoS ONE 7, e34192 (2012).
Comerford, I., Milasta, S., Morrow, V., Milligan, G. & Nibbs, R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur. J. Immunol. 36, 1904–1916 (2006).
Torossian, F. et al. CXCR7 participates in CXCL12-induced CD34+ cell cycling through β-arrestin-dependent Akt activation. Blood 123, 191–202 (2014).
Matti, C. et al. ACKR4 recruits GRK3 prior to β-arrestins but can scavenge chemokines in the absence of β-arrestins. Front. Immunol. 11, 720 (2020).
Saaber, F. et al. ACKR3 regulation of neuronal migration requires ACKR3 phosphorylation, but not β-arrestin. Cell Rep. 26, 1473–1488.e9 (2019).
Montpas, N. et al. Ligand-specific conformational transitions and intracellular transport are required for atypical chemokine receptor 3-mediated chemokine scavenging. J. Biol. Chem. 293, 893–905 (2018).
Schafer, C. T., Chen, Q., Tesmer, J. J. G. & Handel, T. M. Atypical chemokine receptor 3 ‘senses’ CXC chemokine receptor 4 activation through GPCR kinase phosphorylation. Mol. Pharmacol. https://doi.org/10.1124/molpharm.123.000710 (2023).
Zarca, A. et al. Differential involvement of ACKR3 C-tail in β-arrestin recruitment, trafficking and internalization. Cells 10, 618 (2021).
Mackie, D. I. et al. RAMP3 determines rapid recycling of atypical chemokine receptor-3 for guided angiogenesis. Proc. Natl Acad. Sci. USA 116, 24093–24099 (2019).
Borroni, E. M. et al. β-Arrestin-dependent activation of the cofilin pathway is required for the scavenging activity of the atypical chemokine receptor D6. Sci. Signal. 6, ra30 (2013).
Vacchini, A. et al. Control of cytoskeletal dynamics by β-arrestin1/myosin Vb signaling regulates endosomal sorting and scavenging activity of the atypical chemokine receptor ACKR2. Vaccines 8, 542 (2020).
Décaillot, F. M. et al. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J. Biol. Chem. 286, 32188–32197 (2011).
Levoye, A., Balabanian, K., Baleux, F., Bachelerie, F. & Lagane, B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113, 6085–6093 (2009).
Odemis, V. et al. The presumed atypical chemokine receptor CXCR7 signals through G(i/o) proteins in primary rodent astrocytes and human glioma cells. Glia 60, 372–381 (2012).
Fumagalli, A. et al. The atypical chemokine receptor 3 interacts with connexin 43 inhibiting astrocytic gap junctional intercellular communication. Nat. Commun. 11, 4855 (2020).
Sierro, F. et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl Acad. Sci. USA 104, 14759–14764 (2007).
Gerrits, H. et al. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis 46, 235–245 (2008).
Yu, S., Crawford, D., Tsuchihashi, T., Behrens, T. W. & Srivastava, D. The chemokine receptor CXCR7 functions to regulate cardiac valve remodeling. Dev. Dyn. 240, 384–393 (2011).
Gustavsson, M. et al. Structural basis of ligand interaction with atypical chemokine receptor 3. Nat. Commun. 8, 14135 (2017).
Yen, Y.-C. et al. Structures of atypical chemokine receptor 3 reveal the basis for its promiscuity and signaling bias. Sci. Adv. 8, eabn8063 (2022).
Kleist, A. B. et al. Conformational selection guides β-arrestin recruitment at a biased G protein-coupled receptor. Science 377, 222–228 (2022).
Thiriot, A. et al. Differential DARC/ACKR1 expression distinguishes venular from non-venular endothelial cells in murine tissues. BMC Biol. 15, 45 (2017).
Takatsuka, S., Sekiguchi, A., Tokunaga, M., Fujimoto, A. & Chiba, J. Generation of a panel of monoclonal antibodies against atypical chemokine receptor CCX-CKR by DNA immunization. J. Pharmacol. Toxicol. Methods 63, 250–257 (2011).
Bobkov, V. et al. Antibodies targeting chemokine receptors CXCR4 and ACKR3. Mol. Pharmacol. 96, 753–764 (2019).
Hansell, C. A. H. et al. Analysis of lung stromal expression of the atypical chemokine receptor ACKR2 reveals unanticipated expression in murine blood endothelial cells. Eur. J. Immunol. 50, 666–675 (2020).
Hur, J. et al. CD82/KAI1 maintains the dormancy of long-term hematopoietic stem cells through interaction with DARC-expressing macrophages. Cell Stem Cell 18, 508–521 (2016).
Rot, A. et al. Murine bone marrow macrophages and human monocytes do not express atypical chemokine receptor 1. Cell Stem Cell 29, 1013–1015 (2022).
Neote, K., Mak, J. Y., Kolakowski, L. F. & Schall, T. J. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 84, 44–52 (1994).
Horuk, R. et al. Expression of chemokine receptors by subsets of neurons in the central nervous system. J. Immunol. 158, 2882–2890 (1997).
Madigan, J. et al. Chemokine scavenger D6 is expressed by trophoblasts and aids the survival of mouse embryos transferred into allogeneic recipients. J. Immunol. 184, 3202–3212 (2010).
Teoh, P. J. et al. Atypical chemokine receptor ACKR2 mediates chemokine scavenging by primary human trophoblasts and can regulate fetal growth, placental structure, and neonatal mortality in mice. J. Immunol. 193, 5218–5228 (2014).
Thomson, C. A. et al. Expression of the atypical chemokine receptor ACKR4 identifies a novel population of intestinal submucosal fibroblasts that preferentially expresses endothelial cell regulators. J. Immunol. 201, 215–229 (2018).
Bastow, C. R. et al. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc. Natl Acad. Sci. USA 118, e2025763118 (2021).
Zhang, M. et al. Inhibition of fibroblast IL-6 production by ACKR4 deletion alleviates cardiac remodeling after myocardial infarction. Biochem. Biophys. Res. Commun. 547, 139–147 (2021).
Hansell, C. A. H. et al. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood 117, 5413–5424 (2011).
Ford, L. B. et al. Characterization of conventional and atypical receptors for the chemokine CCL2 on mouse leukocytes. J. Immunol. 193, 400–411 (2014).
McKimmie, C. S. et al. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J. Immunol. 181, 3353–3363 (2008).
Radice, E. et al. Marginal zone formation requires ACKR3 expression on B cells. Cell Rep. 32, 107951 (2020).
Kara, E. E. et al. Atypical chemokine receptor 4 shapes activated B cell fate. J. Exp. Med. 215, 801–813 (2018).
Kealy, L. et al. The histone methyltransferase DOT1L is essential for humoral immune responses. Cell Rep. 33, 108504 (2020).
Lau, S. et al. A negative-feedback loop maintains optimal chemokine concentrations for directional cell migration. Nat. Cell Biol. 22, 266–273 (2020).
Klei, T. R. L. et al. Differential interaction between DARC and SDF-1 on erythrocytes and their precursors. Sci. Rep. 9, 16245 (2019).
Gutjahr, J. C. et al. The dimeric form of CXCL12 binds to atypical chemokine receptor 1. Sci. Signal. 14, eabc9012 (2021).
Drury, L. J. et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc. Natl Acad. Sci. USA 108, 17655–17660 (2011).
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Nibbs, R. J., Wylie, S. M., Yang, J., Landau, N. R. & Graham, G. J. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J. Biol. Chem. 272, 32078–32083 (1997).
Bonecchi, R. et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J. Immunol. 172, 4972–4976 (2004).
Fra, A. M. et al. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J. Immunol. 170, 2279–2282 (2003).
Jamieson, T. et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat. Immunol. 6, 403–411 (2005).
Martinez de la Torre, Y. et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur. J. Immunol. 35, 1342–1346 (2005).
Lee, K. M. et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 118, 6220–6229 (2011).
Wilson, G. J. et al. Chemokine receptors coordinately regulate macrophage dynamics and mammary gland development. Dev. Camb. Engl. 147, dev187815 (2020).
Wilson, G. J. et al. Atypical chemokine receptor ACKR2 controls branching morphogenesis in the developing mammary gland. Dev. Camb. Engl. 144, 74–82 (2017).
Chevigné, A. et al. CXCL10 is an agonist of the CC family chemokine scavenger receptor ACKR2/D6. Cancers 13, 1054 (2021).
Groom, J. R. & Luster, A. D. CXCR3 in T cell function. Exp. Cell Res. 317, 620–631 (2011).
Prizant, H. et al. CXCL10+ peripheral activation niches couple preferred sites of Th1 entry with optimal APC encounter. Cell Rep. 36, 109523 (2021).
Duckworth, B. C. et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat. Immunol. 22, 434–448 (2021).
Burns, J. M. et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201–2213 (2006).
Klein, K. R. et al. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev. Cell 30, 528–540 (2014).
Humpert, M.-L., Pinto, D., Jarrossay, D. & Thelen, M. CXCR7 influences the migration of B cells during maturation. Eur. J. Immunol. 44, 694–705 (2014).
Sánchez-Alcañiz, J. A. et al. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron 69, 77–90 (2011).
Schönemeier, B. et al. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J. Comp. Neurol. 510, 207–220 (2008).
Humpert, M.-L. et al. Complementary methods provide evidence for the expression of CXCR7 on human B cells. Proteomics 12, 1938–1948 (2012).
Balabanian, K. et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 280, 35760–35766 (2005).
Boldajipour, B. et al. Control of chemokine-guided cell migration by ligand sequestration. Cell 132, 463–473 (2008).
Dambly-Chaudière, C., Cubedo, N. & Ghysen, A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev. Biol. 7, 23 (2007).
Berahovich, R. D. et al. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology 141, 111–122 (2014).
Alampour-Rajabi, S. et al. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. 29, 4497–4511 (2015).
Chatterjee, M. et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ. Res. 115, 939–949 (2014).
Szpakowska, M. et al. Human herpesvirus 8-encoded chemokine vCCL2/vMIP-II is an agonist of the atypical chemokine receptor ACKR3/CXCR7. Biochem. Pharmacol. 114, 14–21 (2016).
Meyrath, M. et al. The atypical chemokine receptor ACKR3/CXCR7 is a broad-spectrum scavenger for opioid peptides. Nat. Commun. 11, 3033 (2020).
Szpakowska, M. et al. The natural analgesic conolidine targets the newly identified opioid scavenger ACKR3/CXCR7. Signal Transduct. Target. Ther. 6, 209 (2021).
Ikeda, Y., Kumagai, H., Skach, A., Sato, M. & Yanagisawa, M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell 155, 1323–1336 (2013).
Meyrath, M. et al. Proadrenomedullin N-terminal 20 peptides (PAMPs) are agonists of the chemokine scavenger receptor ACKR3/CXCR7. ACS Pharmacol. Transl. Sci. 4, 813–823 (2021).
Sigmund, E. C. et al. Reassessing the adrenomedullin scavenging function of ACKR3 in lymphatic endothelial cells. PLoS ONE 18, e0285597 (2023).
Zabel, B. A. et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J. Immunol. 183, 3204–3211 (2009).
Gosling, J. et al. Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J. Immunol. 164, 2851–2856 (2000).
Townson, J. R. & Nibbs, R. J. B. Characterization of mouse CCX-CKR, a receptor for the lymphocyte-attracting chemokines TECK/mCCL25, SLC/mCCL21 and MIP-3beta/mCCL19: comparison to human CCX-CKR. Eur. J. Immunol. 32, 1230–1241 (2002).
Misslitz, A. et al. Thymic T cell development and progenitor localization depend on CCR7. J. Exp. Med. 200, 481–491 (2004).
Ueno, T. et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J. Exp. Med. 200, 493–505 (2004).
Kurobe, H. et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 24, 165–177 (2006).
Benz, C., Heinzel, K. & Bleul, C. C. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur. J. Immunol. 34, 3652–3663 (2004).
Braun, A. et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12, 879–887 (2011).
Tal, O. et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Warnock, R. A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer’s patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Förster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Ulvmar, M. H. et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 15, 623–630 (2014). This study demonstrates that ACKR is expressed on ceiling lymphatic vessels in the lymph node subcapsular sinus and governs the formation of interfollicular extracellular CCL21 gradients that are required for efficient migration of DCs from the lymph node subcapsular sinus to the T cell zone.
Bryce, S. A. et al. ACKR4 on stromal cells scavenges CCL19 to enable CCR7-dependent trafficking of APCs from inflamed skin to lymph nodes. J. Immunol. 196, 3341–3353 (2016). This study is, to our knowledge, the first to determine that ACKR4 in the skin modulates CCR7 ligand chemokines and is required for efficient recruitment of DCs out of the skin.
Heinzel, K., Benz, C. & Bleul, C. C. A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc. Natl Acad. Sci. USA 104, 8421–8426 (2007).
Rode, I. & Boehm, T. Regenerative capacity of adult cortical thymic epithelial cells. Proc. Natl Acad. Sci. USA 109, 3463–3468 (2012).
Bunting, M. D. et al. CCX-CKR deficiency alters thymic stroma impairing thymocyte development and promoting autoimmunity. Blood 121, 118–128 (2013).
Comerford, I. et al. An immune paradox: how can the same chemokine axis regulate both immune tolerance and activation?: CCR6/CCL20: a chemokine axis balancing immunological tolerance and inflammation in autoimmune disease. BioEssays 32, 1067–1076 (2010).
Schutyser, E., Struyf, S. & Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 (2003).
Matti, C. et al. CCL20 is a novel ligand for the scavenging atypical chemokine receptor 4. J. Leukoc. Biol. 107, 1137–1154 (2020).
Meyrath, M., Reynders, N., Uchański, T., Chevigné, A. & Szpakowska, M. Systematic reassessment of chemokine-receptor pairings confirms CCL20 but not CXCL13 and extends the spectrum of ACKR4 agonists to CCL22. J. Leukoc. Biol. 109, 373–376 (2021).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Villares, R. et al. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur. J. Immunol. 39, 1671–1681 (2009).
Lin, Y.-L., Ip, P.-P. & Liao, F. CCR6 deficiency impairs IgA production and dysregulates antimicrobial peptide production, altering the intestinal flora. Front. Immunol. 8, 805 (2017).
Reimer, D. et al. Early CCR6 expression on B cells modulates germinal centre kinetics and efficient antibody responses. Immunol. Cell Biol. 95, 33–41 (2017).
Yamamoto, J. et al. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J. Leukoc. Biol. 68, 568–574 (2000).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646 (2007).
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Soler, D., Humphreys, T. L., Spinola, S. M. & Campbell, J. J. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood 101, 1677–1682 (2003).
Trifari, S., Kaplan, C. D., Tran, E. H., Crellin, N. K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10, 864–871 (2009).
Mariani, M., Lang, R., Binda, E., Panina-Bordignon, P. & D’Ambrosio, D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 34, 231–240 (2004).
Verkaar, F. et al. Chemokine cooperativity is caused by competitive glycosaminoglycan binding. J. Immunol. 192, 3908–3914 (2014).
Davenport, A. P. et al. International union of basic and clinical pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. 65, 967–986 (2013).
Bachelerie, F. et al. An atypical addition to the chemokine receptor nomenclature: IUPHAR review 15. Br. J. Pharmacol. 172, 3945–3949 (2015).
Kapas, S., Catt, K. J. & Clark, A. J. Cloning and expression of cDNA encoding a rat adrenomedullin receptor. J. Biol. Chem. 270, 25344–25347 (1995).
Kennedy, S. P. et al. Expression of the rat adrenomedullin receptor or a putative human adrenomedullin receptor does not correlate with adrenomedullin binding or functional response. Biochem. Biophys. Res. Commun. 244, 832–837 (1998).
Kwon, H.-B. et al. The orphan G-protein coupled receptor 182 is a negative regulator of definitive hematopoiesis through leukotriene B4 signaling. ACS Pharmacol. Transl. Sci. 3, 676–689 (2020).
Le Mercier, A. et al. GPR182 is an endothelium-specific atypical chemokine receptor that maintains hematopoietic stem cell homeostasis. Proc. Natl Acad. Sci. USA 118, e2021596118 (2021). This study is, to our knowledge, the first to identify that GPR182 is an atypical chemokine receptor that regulates levels of CXCL10, CXCL12 and CXCL13 in vivo and influences HSC homoeostasis.
Torphy, R. J. et al. GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models. Nat. Commun. 13, 97 (2022).
Melgrati, S. et al. GPR182 is a broadly scavenging atypical chemokine receptor influencing T-independent immunity. Front. Immunol. 14, 1242531 (2023).
Biber, K., Zuurman, M. W., Homan, H. & Boddeke, H. W. G. M. Expression of L-CCR in HEK 293 cells reveals functional responses to CCL2, CCL5, CCL7, and CCL8. J. Leukoc. Biol. 74, 243–251 (2003).
Leick, M. et al. CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAM-B. Immunology 129, 536–546 (2010).
Catusse, J. et al. Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Mol. Cancer 9, 297 (2010).
Su, Z. et al. Studies with neutralizing antibodies suggest CXCL8-mediated neutrophil activation is independent of C-C motif chemokine receptor-like 2 (CCRL2) ligand binding function. PLoS ONE 18, e0280590 (2023).
Zabel, B. A. et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 205, 2207–2220 (2008).
Fischer, T. F. & Beck-Sickinger, A. G. Chemerin — exploring a versatile adipokine. Biol. Chem. 403, 625–642 (2022).
Al Delbany, D. et al. Expression of CCRL2 inhibits tumor growth by concentrating chemerin and inhibiting neoangiogenesis. Cancers 13, 5000 (2021).
De Henau, O. et al. Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 11, e0164179 (2016).
Degroot, G.-N., Lepage, V., Parmentier, M. & Springael, J.-Y. The atypical chemerin receptor GPR1 displays different modes of interaction with β-arrestins in humans and mice with important consequences on subcellular localization and trafficking. Cells 11, 1037 (2022).
Del Prete, A. et al. The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood 130, 1223–1234 (2017).
Li, X. X., Lee, J. D., Kemper, C. & Woodruff, T. M. The complement receptor C5aR2: a powerful modulator of innate and adaptive immunity. J. Immunol. 202, 3339–3348 (2019).
Chen, J. et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 19, 541–555 (2011).
Lev, S. et al. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell. Biol. 19, 2278–2288 (1999).
Zeng, W. et al. CCL18 signaling from tumor-associated macrophages activates fibroblasts to adopt a chemoresistance-inducing phenotype. Oncogene 42, 224–237 (2023).
Jiang, X. et al. CCL18-NIR1 promotes oral cancer cell growth and metastasis by activating the JAK2/STAT3 signaling pathway. BMC Cancer 20, 632 (2020).
Campanella, G. S. V., Colvin, R. A. & Luster, A. D. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 5, e12700 (2010).
Korniejewska, A., McKnight, A. J., Johnson, Z., Watson, M. L. & Ward, S. G. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 132, 503–515 (2011).
Lasagni, L. et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 197, 1537–1549 (2003).
Struyf, S. et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood 117, 480–488 (2011).
Gray, A. L. et al. Chemokine CXCL4 interactions with extracellular matrix proteoglycans mediate widespread immune cell recruitment independent of chemokine receptors. Cell Rep. 42, 111930 (2023).
D’Uonnolo, G. et al. The extended N-terminal domain confers atypical chemokine receptor properties to CXCR3-B. Front. Immunol. 13, 868579 (2022). This study identifies the atypical chemokine receptor-like properties of CXCR3-B, determining that its extended N-terminus prevents G protein coupling and modifies its subcellular localization.
Smith, J. S. et al. C-X-C motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways. Mol. Pharmacol. 92, 136–150 (2017).
Sfriso, P. et al. Epithelial CXCR3-B regulates chemokines bioavailability in normal, but not in Sjogren’s syndrome, salivary glands. J. Immunol. 176, 2581–2589 (2006).
Borroni, E. M., Savino, B., Bonecchi, R. & Locati, M. Chemokines sound the alarmin: the role of atypical chemokine in inflammation and cancer. Semin. Immunol. 38, 63–71 (2018).
Crawford, K. S. & Volkman, B. F. Prospects for targeting ACKR1 in cancer and other diseases. Front. Immunol. 14, 1111960 (2023).
Koch, C. & Engele, J. Functions of the CXCL12 receptor ACKR3/CXCR7 — what has been perceived and what has been overlooked. Mol. Pharmacol. 98, 577–585 (2020).
Gowhari Shabgah, A. et al. The role of atypical chemokine receptor D6 (ACKR2) in physiological and pathological conditions; friend, foe, or both? Front. Immunol. 13, 861931 (2022).
Tavares, L. P. et al. ACKR2 contributes to pulmonary dysfunction by shaping CCL5:CCR5-dependent recruitment of lymphocytes during influenza A infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L655–L670 (2020).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013). This seminal study provides the first direct visualization of an extracellular chemokine gradient, which was shown to govern DC sensing of lymphatic vessels in the skin.
Comerford, I. et al. The atypical chemokine receptor CCX-CKR scavenges homeostatic chemokines in circulation and tissues and suppresses Th17 responses. Blood 116, 4130–4140 (2010).
Jafarnejad, M., Zawieja, D. C., Brook, B. S., Nibbs, R. J. B. & Moore, J. E. A novel computational model predicts key regulators of chemokine gradient formation in lymph nodes and site-specific roles for CCL19 and ACKR4. J. Immunol. 199, 2291–2304 (2017).
Friess, M. C. et al. Mechanosensitive ACKR4 scavenges CCR7 chemokines to facilitate T cell de-adhesion and passive transport by flow in inflamed afferent lymphatics. Cell Rep. 38, 110334 (2022). This study demonstrates how ACKR4 regulates chemokine deposition in lymphatic collector vessels and facilitates T cell migration in afferent lymph.
Werth, K. et al. Expression of ACKR4 demarcates the ‘peri-marginal sinus,’ a specialized vascular compartment of the splenic red pulp. Cell Rep. 36, 109346 (2021).
Marchetti, L. et al. ACKR1 favors transcellular over paracellular T‐cell diapedesis across the blood‐brain barrier in neuroinflammation in vitro. Eur. J. Immunol. 52, 161–177 (2022).
Girbl, T. et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 49, 1062–1076.e6 (2018). This study elegantly determines how ACKR1, CXCL1 and CXCL2 are compartmentalized on endothelial cells to facilitate the stages of neutrophil diapedesis.
Wang, H. et al. The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood 119, 465–468 (2012).
Eckert, N., Werth, K., Willenzon, S., Tan, L. & Förster, R. B cell hyperactivation in an Ackr4-deficient mouse strain is not caused by lack of ACKR4 expression. J. Leukoc. Biol. 107, 1155–1166 (2020).
Zhang, Y. et al. Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift. Nat. Commun. 13, 2460 (2022).
Merz, L. E. et al. Absolute neutrophil count by Duffy status among healthy Black and African American adults. Blood Adv. 7, 317–320 (2023).
Duchene, J. et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nat. Immunol. 18, 753–761 (2017). This study identifies the mechanism that links the absence of ACKR1 on erythroid cells with neutropenia.
Charles, B. A. et al. Analyses of genome wide association data, cytokines, and gene expression in African-Americans with benign ethnic neutropenia. PLoS ONE 13, e0194400 (2018).
Miyabe, Y., Miyabe, C., Mani, V., Mempel, T. R. & Luster, A. D. Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation. Sci. Immunol. 4, eaav5951 (2019).
Martin, C. et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 (2003).
Barkaway, A. et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 54, 1494–1510.e7 (2021).
Rot, A. et al. Cell-autonomous regulation of neutrophil migration by the D6 chemokine decoy receptor. J. Immunol. 190, 6450–6456 (2013).
Massara, M. et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat. Commun. 9, 676 (2018). This study identifies the important contribution of ACKR2 expressed by haematopoietic progenitors in the release of neutrophils in the context of breast cancer and melanoma.
Pashover-Schallinger, E. et al. The atypical chemokine receptor D6 controls macrophage efferocytosis and cytokine secretion during the resolution of inflammation. FASEB J. 26, 3891–3900 (2012).
Aswad, M., Assi, S., Schif-Zuck, S. & Ariel, A. CCL5 promotes resolution-phase macrophage reprogramming in concert with the atypical chemokine receptor D6 and apoptotic polymorphonuclear cells. J. Immunol. 199, 1393–1404 (2017).
Heesen, M. et al. Cloning and chromosomal mapping of an orphan chemokine receptor: mouse RDC1. Immunogenetics 47, 364–370 (1998).
Ngamsri, K.-C. et al. The pivotal role of CXCR7 in stabilization of the pulmonary epithelial barrier in acute pulmonary inflammation. J. Immunol. 198, 2403–2413 (2017).
Cruz-Orengo, L. et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med. 208, 327–339 (2011).
Ameti, R. et al. Characterization of a chimeric chemokine as a specific ligand for ACKR3. J. Leukoc. Biol. 104, 391–400 (2018).
Konrad, F. M., Meichssner, N., Bury, A., Ngamsri, K.-C. & Reutershan, J. Inhibition of SDF-1 receptors CXCR4 and CXCR7 attenuates acute pulmonary inflammation via the adenosine A2B-receptor on blood cells. Cell Death Dis. 8, e2832 (2017).
Ngamsri, K.-C. et al. CXCR4 and CXCR7 inhibition ameliorates the formation of platelet-neutrophil complexes and neutrophil extracellular traps through Adora2b signaling. Int. J. Mol. Sci. 22, 13576 (2021).
Calì, B. et al. Atypical CXCL12 signaling enhances neutrophil migration by modulating nuclear deformability. Sci. Signal. 15, eabk2552 (2022).
Gencer, S. et al. Endothelial ACKR3 drives atherosclerosis by promoting immune cell adhesion to vascular endothelium. Basic Res. Cardiol. 117, 30 (2022).
Peiper, S. C. et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J. Exp. Med. 181, 1311–1317 (1995).
Szabo, M. C., Soo, K. S., Zlotnik, A. & Schall, T. J. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J. Biol. Chem. 270, 25348–25351 (1995).
Gardner, L., Patterson, A. M., Ashton, B. A., Stone, M. A. & Middleton, J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem. Biophys. Res. Commun. 321, 306–312 (2004).
Spaan, A. N. et al. Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18, 363–370 (2015).
Bolton, M. J. & Garry, R. F. Sequence similarity between the erythrocyte binding domain 1 of the Plasmodium vivax Duffy binding protein and the V3 loop of HIV-1 strain MN reveals binding residues for the Duffy antigen receptor for chemokines. Virol. J. 8, 45 (2011).
Chitnis, C. E. & Miller, L. H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 180, 497–506 (1994).
Nibbs, R. J., Wylie, S. M., Pragnell, I. B. & Graham, G. J. Cloning and characterization of a novel murine beta chemokine receptor, D6. Comparison to three other related macrophage inflammatory protein-1alpha receptors, CCR-1, CCR-3, and CCR-5. J. Biol. Chem. 272, 12495–12504 (1997).
Nibbs, R. J., Yang, J., Landau, N. R., Mao, J. H. & Graham, G. J. LD78beta, a non-allelic variant of human MIP-1alpha (LD78alpha), has enhanced receptor interactions and potent HIV suppressive activity. J. Biol. Chem. 274, 17478–17483 (1999).
Goncharuk, M. V. et al. Purification of native CCL7 and its functional interaction with selected chemokine receptors. Protein Expr. Purif. 171, 105617 (2020).
Acknowledgements
I.C. is supported by a Senior Research Fellowship from MS Australia.
Author information
Authors and Affiliations
Contributions
I.C. contributed to the writing, discussion, review and editing of the manuscript; S.R.McC. contributed to the discussion, review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks P. von Hundelshausen, J. Duchene and R. Bonecchi for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Comerford, I., McColl, S.R. Atypical chemokine receptors in the immune system. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01025-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01025-5