Abstract
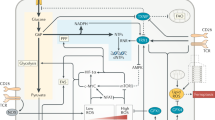
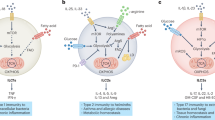
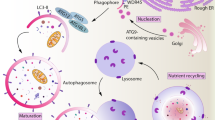
Initiating and maintaining optimal immune responses requires high levels of protein synthesis, folding, modification and trafficking in leukocytes, which are processes orchestrated by the endoplasmic reticulum. Importantly, diverse extracellular and intracellular conditions can compromise the protein-handling capacity of this organelle, inducing a state of ‘endoplasmic reticulum stress’ that activates the unfolded protein response (UPR). Emerging evidence shows that physiological or pathological activation of the UPR can have effects on immune cell survival, metabolism, function and fate. In this Review, we discuss the canonical role of the adaptive UPR in immune cells and how dysregulation of this pathway in leukocytes contributes to diverse pathologies such as cancer, autoimmunity and metabolic disorders. Furthermore, we provide an overview as to how pharmacological approaches that modulate the UPR could be harnessed to control or activate immune cell function in disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Xu, C. & Ng, D. T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 16, 742–752 (2015).
Shiu, R. P., Pouyssegur, J. & Pastan, I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteins in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc. Natl Acad. Sci. USA 74, 3840–3844 (1977).
Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988).
Mori, K. The unfolded protein response: the dawn of a new field. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 91, 469–480 (2015).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Celli, J. & Tsolis, R. M. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat. Rev. Microbiol. 13, 71–82 (2015).
Tavernier, S. J., Lambrecht, B. N. & Janssens, S. The unfolded protein response in the immune cell development: putting the caretaker in the driving seat. Curr. Top. Microbiol. Immunol. 414, 45–721 (2018).
Bettigole, S. E. & Glimcher, L. H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138 (2015).
Martinon, F., Chen, X., Lee, A. H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010). This study highlights the importance of XBP1s-mediated transcriptional regulation for optimizing macrophage polarization.
Qiu, Q. et al. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J. 32, 2477–2490 (2013).
Keestra-Gounder, A. M. et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532, 394–397 (2016).
Sweet, L. A., Kuss-Duerkop, S. K. & Keestra-Gounder, A. M. IRE1alpha-driven inflammation promotes clearance of citrobacter rodentium infection. Infect. Immun. 90, e0048121 (2022).
Iwasaki, Y. et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes 63, 152–161 (2014).
Hu, F. et al. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur. J. Immunol. 41, 1086–1097 (2011).
Moretti, J. et al. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171, 809–823 (2017).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Zhang, D. et al. A non-canonical cGAS–STING–PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat. Cell Biol. 24, 766–782 (2022).
Chaudhary, V. et al. Chronic activation of pDCs in autoimmunity is linked to dysregulated ER stress and metabolic responses. J. Exp. Med. 219, e20221085 (2022).
Goodall, J. C. et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl Acad. Sci. USA 107, 17698–17703 (2010).
Marquez, S. et al. Endoplasmic reticulum stress sensor IRE1alpha enhances IL-23 expression by human dendritic cells. Front. Immunol. 8, 639 (2017).
Mogilenko, D. A. et al. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell 178, 263 (2019).
Rosen, D. A. et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11, eaau5266 (2019).
Govindarajan, S. et al. Stabilization of cytokine mRNAs in iNKT cells requires the serine–threonine kinase IRE1alpha. Nat. Commun. 9, 5340 (2018).
Chopra, S. et al. IRE1alpha-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science 365, eaau6499 (2019).
Park, J. Y., Pillinger, M. H. & Abramson, S. B. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin. Immunol. 119, 229–240 (2006).
Yan, D., Wang, H. W., Bowman, R. L. & Joyce, J. A. STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1alpha activation. Cell Rep. 16, 2914–2927 (2016).
Dong, H. et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol. 20, 865–878 (2019). This study shows how UPR signalling regulates cMYC-mediated transcriptional and metabolic reprogramming of NK cells.
Medel, B. et al. IRE1alpha activation in bone marrow-derived dendritic cells modulates innate recognition of melanoma cells and favors CD8(+) T cell priming. Front. Immunol. 9, 3050 (2018).
Guttman, O. et al. Antigen-derived peptides engage the ER stress sensor IRE1alpha to curb dendritic cell cross-presentation. J. Cell Biol. 221, e202111068 (2022).
Brunsing, R. et al. B- and T-cell development both involve activity of the unfolded protein response pathway. J. Biol. Chem. 283, 17954–17961 (2008).
Pino, S. C. et al. Protein kinase C signaling during T cell activation induces the endoplasmic reticulum stress response. Cell Stress Chaperones 13, 421–434 (2008).
Berry, C. T. et al. BCR-induced Ca(2+) signals dynamically tune survival, metabolic reprogramming, and proliferation of naive B cells. Cell Rep. 31, 107474 (2020).
Michalak, M., Robert Parker, J. M. & Opas, M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32, 269–278 (2002).
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Wang, Z. V. et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156, 1179–1192 (2014).
Song, M. et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428 (2018).
Cao, Y. et al. ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat. Commun. 10, 1280 (2019). This study shows that a tumour-derived factor alters the protein-folding machinery in T cells, which in turn leads to T cell dysfunction.
Kamimura, D. & Bevan, M. J. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+T cell differentiation during acute infection. J. Immunol. 181, 5433–5441 (2008).
Pramanik, J. et al. Genome-wide analyses reveal the IRE1a–XBP1 pathway promotes T helper cell differentiation by resolving secretory stress and accelerating proliferation. Genome Med. 10, 76 (2018).
Bettigole, S. E. et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 16, 829–837 (2015).
Brucklacher-Waldert, V. et al. Cellular stress in the context of an inflammatory environment supports TGF-beta-independent T helper-17 differentiation. Cell Rep. 19, 2357–2370 (2017).
Denzel, M. S. & Antebi, A. Hexosamine pathway and (ER) protein quality control. Curr. Opin. Cell Biol. 33, 14–18 (2015).
Yang, X. et al. ATF4 regulates CD4(+) T cell immune responses through metabolic reprogramming. Cell Rep. 23, 1754–1766 (2018).
Franco, A., Almanza, G., Burns, J. C., Wheeler, M. & Zanetti, M. Endoplasmic reticulum stress drives a regulatory phenotype in human T-cell clones. Cell Immunol. 266, 1–6 (2010).
van Anken, E., Bakunts, A., Hu, C. A., Janssens, S. & Sitia, R. Molecular evaluation of endoplasmic reticulum homeostasis meets humoral immunity. Trends Cell Biol. 31, 529–541 (2021).
Todd, D. J. et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206, 2151–2159 (2009).
Iwakoshi, N. N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 (2003).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Aragon, I. V., Barrington, R. A., Jackowski, S., Mori, K. & Brewer, J. W. The specialized unfolded protein response of B lymphocytes: ATF6alpha-independent development of antibody-secreting B cells. Mol. Immunol. 51, 347–355 (2012).
Gass, J. N., Jiang, H. Y., Wek, R. C. & Brewer, J. W. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol. Immunol. 45, 1035–1043 (2008).
McGehee, A. M. et al. XBP-1-deficient plasmablasts show normal protein folding but altered glycosylation and lipid synthesis. J. Immunol. 183, 3690–3699 (2009).
Hu, C. C., Dougan, S. K., McGehee, A. M., Love, J. C. & Ploegh, H. L. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 28, 1624–1636 (2009).
Benhamron, S. et al. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur. J. Immunol. 44, 867–876 (2014).
Riera-Domingo, C. et al. Immunity, hypoxia, and metabolism — the menage a trois of cancer: implications for immunotherapy. Physiol. Rev. 100, 1–102 (2020).
Boedtkjer, E. & Pedersen, S. F. The acidic tumor microenvironment as a driver of cancer. Annu. Rev. Physiol. 82, 103–126 (2020).
Cubillos-Ruiz, J. R. et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015). This study provides a mechanistic understanding of how XBP1s signalling is engaged in tumour-infiltrating dendritic cells, which impairs their antigen presentation and maturation.
Lu, Y. et al. Reactivation of dysfunctional dendritic cells by a stress-relieving nanosystem resets anti-tumor immune landscape. Nano Today 43, 101416 (2022).
Zhu, C. et al. Tim-3 adaptor protein Bat3 is a molecular checkpoint of T cell terminal differentiation and exhaustion. Sci. Adv. 7, eabd2710 (2021).
Tian, S., Liu, Z., Donahue, C., Falo, L. D. Jr & You, Z. Genetic targeting of the active transcription factor XBP1s to dendritic cells potentiates vaccine-induced prophylactic and therapeutic antitumor immunity. Mol. Ther. 20, 432–442 (2012).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Ma, X. et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156 (2019).
Hurst, K. E. et al. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8(+) T cells. Cancer Immunol. Res. 7, 476–486 (2019).
Feng, Z.-Z. et al. ER stress and its PERK branch enhance TCR-induced activation in regulatory T cells. Biochem. Biophys. Res. Commun. 563, 8–14 (2021).
Xian, S. et al. The unfolded protein response links tumor aneuploidy to local immune dysregulation. EMBO Rep. 22, e52509 (2021).
Zhao, Y. et al. XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal. Transduct. Target. Ther. 6, 357 (2021).
Di Conza, G. et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat. Immunol. 22, 1403–1415 (2021).
Raines, L. N. et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 23, 431–445 (2022). Di Conza et al. and Raines et al. show how UPR signalling pathways instruct functional and metabolic repogramming of tumour-associated macrophages to support a pro-tumorigenic phenotype.
Batista, A. et al. IRE1alpha regulates macrophage polarization, PD-L1 expression, and tumor survival. PLoS Biol. 18, e3000687 (2020).
Gilardini Montani, M. S. et al. KSHV infection skews macrophage polarisation towards M2-like/TAM and activates Ire1 alpha-XBP1 axis up-regulating pro-tumorigenic cytokine release and PD-L1 expression. Br. J. Cancer 123, 298–306 (2020).
Liu, P. S. et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Mandula, J. K. & Rodriguez, P. C. Tumor-related stress regulates functional plasticity of MDSCs. Cell Immunol. 363, 104312 (2021).
Mohamed, E. et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 52, 668–682 (2020). This study shows that tumour-infiltrating MDSCs upregulate PERK to sustain their metabolic fitness and immunosuppressive activities.
Condamine, T. et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 1, eaaf8943 (2016).
Tcyganov, E. N. et al. Distinct mechanisms govern populations of myeloid-derived suppressor cells in chronic viral infection and cancer. J. Clin. Invest. 131, e145971 (2021).
McAlpine, C. S. & Werstuck, G. H. The development and progression of atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc. Hematol. Disord. Drug Targets 13, 158–164 (2013).
Tabas, I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107, 839–850 (2010).
Vega, H., Agellon, L. B. & Michalak, M. The rise of proteostasis promoters. IUBMB Life 68, 943–954 (2016).
Erbay, E. et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 (2009).
Tufanli, O. et al. Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc. Natl Acad. Sci. USA 114, E1395–E1404 (2017).
Shan, B. et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 18, 519–529 (2017). This study shows the pathological role of IRE1a activation in obesity, by skewing adipose tissue macrophages towards a pro-inflammatory phenotype that worsens disease progression.
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Petkevicius, K. et al. Accelerated phosphatidylcholine turnover in macrophages promotes adipose tissue inflammation in obesity. eLife 8, e47990 (2019).
Van Campenhout, S. et al. Myeloid-specific IRE1alpha deletion reduces tumour development in a diabetic, non-alcoholic steatohepatitis-induced hepatocellular carcinoma mouse model. Metabolism 107, 154220 (2020).
LaMarche, N. M. et al. Distinct iNKT cell populations use IFNgamma or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab. 32, 243–258 (2020).
Gonnella, R. et al. IRE1 alpha/XBP1 axis sustains primary effusion lymphoma cell survival by promoting cytokine release and STAT3 activation. Biomedicines 9, 118 (2021).
Chen, C. & Zhang, X. IRE1alpha-XBP1 pathway promotes melanoma progression by regulating IL-6/STAT3 signaling. J. Transl. Med. 15, 42 (2017).
Hedrich, C. M. et al. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc. Natl Acad. Sci. USA 111, 13457–13462 (2014).
Hutchins, A. P., Diez, D. & Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief. Funct. Genomics 12, 489–498 (2013).
Liu, Y. et al. Role for the endoplasmic reticulum stress sensor IRE1alpha in liver regenerative responses. J. Hepatol. 62, 590–598 (2015).
Chadwick, S. R. & Lajoie, P. Endoplasmic reticulum stress coping mechanisms and lifespan regulation in health and diseases. Front. Cell Dev. Biol. 7, 84 (2019).
Taylor, R. C. & Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447 (2013).
Denzel, M. S. et al. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell 156, 1167–1178 (2014).
Singh, J. & Aballay, A. Endoplasmic reticulum stress caused by lipoprotein accumulation suppresses immunity against bacterial pathogens and contributes to immunosenescence. mBio 8, e00778–17 (2017).
Martinez, G., Duran-Aniotz, C., Cabral-Miranda, F., Vivar, J. P. & Hetz, C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16, 615–623 (2017).
Fernandez, D., Geisse, A., Bernales, J. I., Lira, A. & Osorio, F. The unfolded protein response in immune cells as an emerging regulator of neuroinflammation. Front. Aging Neurosci. 13, 682633 (2021).
Ulland, T. K. et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663 (2017).
Ulland, T. K. & Colonna, M. TREM2 — a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14, 667–675 (2018).
Nugent, A. A. et al. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105, 837–854.e9 (2020).
Wang, Y. W. et al. Mild endoplasmic reticulum stress ameliorates lipopolysaccharide-induced neuroinflammation and cognitive impairment via regulation of microglial polarization. J. Neuroinflammation 14, 233 (2017).
Xu, Y. et al. The E3 ligase Hrd1 stabilizes Tregs by antagonizing inflammatory cytokine-induced ER stress response. JCI Insight 4, e121887 (2019).
Todd, D. J., Lee, A. H. & Glimcher, L. H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 8, 663–674 (2008).
Wu, B. et al. Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation. Nat. Immunol. 22, 1551–1562 (2021).
Jadeja, S. D., Vaishnav, J., Bharti, A. H. & Begum, R. Elevated X-box binding protein1 splicing and interleukin-17A expression are associated with active generalized vitiligo in Gujarat population. Front. Immunol. 12, 801724 (2021).
Rees, W. D. et al. Enteroids derived from inflammatory bowel disease patients display dysregulated endoplasmic reticulum stress pathways, leading to differential inflammatory responses and dendritic cell maturation. J. Crohns Colitis 14, 948–961 (2020).
Coleman, O. I. & Haller, D. ER stress and the UPR in shaping intestinal tissue homeostasis and immunity. Front. Immunol. 10, 2825 (2019).
Huang, C., Hedl, M., Ranjan, K. & Abraham, C. LACC1 required for NOD2-induced, ER stress-mediated innate immune outcomes in human macrophages and lacc1 risk variants modulate these outcomes. Cell Rep. 29, 4525–4539 (2019).
Mhaille, A. N. et al. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 67, 200–211 (2008).
Hetz, C., Chevet, E. & Harding, H. P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12, 703–719 (2013).
Hetz, C., Axten, J. M. & Patterson, J. B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 15, 764–775 (2019).
Chen, D. et al. IRE1alpha inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic–ischemic brain injury in rats. J. Neuroinflammation 15, 32 (2018).
Volkmann, K. et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 286, 12743–12755 (2011).
Mimura, N. et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood 119, 5772–5781 (2012).
Tang, C. H. et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J. Clin. Invest. 124, 2585–2598 (2014). This study shows that genetic and pharmacological inhibition of XBP1 decreases the survival of B cell lymphoma cells, by impairing BCR signalling.
Sheng, X. et al. IRE1alpha-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun. 10, 323 (2019).
Ali, M. M. et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 30, 894–905 (2011).
Tang, X., Teder, T., Samuelsson, B. & Haeggstrom, J. Z. The IRE1alpha inhibitor KIRA6 blocks leukotriene biosynthesis in human phagocytes. Front. Pharmacol. 13, 806240 (2022).
Rufo, N. et al. Stress-induced inflammation evoked by immunogenic cell death is blunted by the IRE1alpha kinase inhibitor KIRA6 through HSP60 targeting. Cell Death Differ. 29, 230–245 (2022).
Morita, S. et al. Targeting ABL-IRE1alpha signaling spares ER-stressed pancreatic beta cells to reverse autoimmune diabetes. Cell Metab. 25, 883–897 (2017).
Harnoss, J. M. et al. Disruption of IRE1alpha through its kinase domain attenuates multiple myeloma. Proc. Natl Acad. Sci. USA 116, 16420–16429 (2019).
Madhavan, A. et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 13, 608 (2022).
Grandjean, J. M. D. et al. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat. Chem. Biol. 16, 1052–1061 (2020).
Axten, J. M. et al.Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 55, 7193–7207 (2012).
Shi, Z. et al. Activation of the PERK–ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci. Rep. 9, 3210 (2019).
Moreno, J. A. et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138 (2013).
Radford, H., Moreno, J. A., Verity, N., Halliday, M. & Mallucci, G. R. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 130, 633–642 (2015).
Sen, T., Gupta, R., Kaiser, H. & Sen, N. Activation of PERK elicits memory impairment through inactivation of CREB and downregulation of PSD95 after traumatic brain injury. J. Neurosci. 37, 5900–5911 (2017).
Grande, V. et al. PERK inhibition delays neurodegeneration and improves motor function in a mouse model of Marinesco–Sjogren syndrome. Hum. Mol. Genet. 27, 2477–2489 (2018).
Rojas-Rivera, D. et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 24, 1100–1110 (2017).
Yu, Q. et al. Type I interferons mediate pancreatic toxicities of PERK inhibition. Proc. Natl Acad. Sci. USA 112, 15420–15425 (2015).
Smith, A. L. et al. Discovery of 1H-pyrazol-3(2H)-ones as potent and selective inhibitors of protein kinase R-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 58, 1426–1441 (2015).
Yousuf, M. S. et al. Endoplasmic reticulum stress in the dorsal root ganglia regulates large-conductance potassium channels and contributes to pain in a model of multiple sclerosis. FASEB J. 34, 12577–12598 (2020).
Sidrauski, C. et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife 2, e00498 (2013).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl. Med. 10, eaar2036 (2018).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Kars, M. et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010).
Qi, X., Hosoi, T., Okuma, Y., Kaneko, M. & Nomura, Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol. Pharmacol. 66, 899–908 (2004).
Mizukami, T. et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J. Vasc. Surg. 52, 1580–1586 (2010).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993).
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Zhou, J. et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl Acad. Sci. USA 103, 14343–14348 (2006).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Han, D. et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 (2009).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Mukherjee, D., Bercz, L. S., Torok, M. A. & Mace, T. A. Regulation of cellular immunity by activating transcription factor 4. Immunol. Lett. 228, 24–34 (2020).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Osorio, F., Lambrecht, B. N. & Janssens, S. Antigen presentation unfolded: identifying convergence points between the UPR and antigen presentation pathways. Curr. Opin. Immunol. 52, 100–107 (2018).
Zhou, Y. et al. The roles of endoplasmic reticulum in NLRP3 inflammasome activation. Cells 9, 1219 (2020).
Bainter, W. et al. Combined immunodeficiency due to a mutation in the gamma1 subunit of the coat protein I complex. J. Clin. Invest. 131, e140494 (2021).
Ono, S. J., Liou, H. C., Davidon, R., Strominger, J. L. & Glimcher, L. H. Human X-box-binding protein 1 is required for the transcription of a subset of human class II major histocompatibility genes and forms a heterodimer with c-fos. Proc. Natl Acad. Sci. USA 88, 4309–4312 (1991).
Price, B. D., Mannheim-Rodman, L. A. & Calderwood, S. K. Brefeldin A, thapsigargin, and AIF4 stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J. Cell Physiol. 152, 545–552 (1992).
Koditz, J. et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 110, 3610–3617 (2007).
Rouschop, K. M. et al. PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl Acad. Sci. USA 110, 4622–4627 (2013).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Fu, S. et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011).
Rong, X. et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18, 685–697 (2013).
Volmer, R., van der Ploeg, K. & Ron, D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl Acad. Sci. USA 110, 4628–4633 (2013). This study shows that IRE1 and PERK can sense disbalanced lipid saturation within the ER membrane through their ER-spanning transmembrane domains.
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Acknowledgements
P.-C.H. was supported in part by SNSF project grants (31003A_182470), Helmut Horten Stifung, Cancer Research Institute Lloyd J. Old STAR Award and the ERC Starting Grant (802773-MitoGuide). S.C.-C.H. was supported by American Cancer Society Research Scholar Grant (RSG-22-135-01-IBCD), Melanoma Research Foundation Career Development Award, Andrew McDonough B+ Foundation Grant Award, Cancer Research Institute CLIP Investigator Award, VeloSano Pilot Award, Case GI SPORE DRP Grant (5P50CA150964-08) and Case Comprehensive Cancer Center American Cancer Society Pilot Grants (IRG-91-022-19, IRG-16-186-21). J.C.-R. has been supported in part by US Department of Defense Grants OC190443, OC200166 and OC200224; Stand Up to Cancer Grants SU2C-AACR-IRG-03-16 and SU2C-AACR-PS-24; National Institutes of Health Grants R01NS114653 and R21CA248106 and The Mark Foundation for Cancer Research ASPIRE Award.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
G.D.C. is employed at iOnctura SA. P.-C.H. serves as a scientific advisor for Elixiron Immunotherapeutics and is a co-founder of Pilatus Biosciences. J.R.C.-R. serves as scientific consultant for NextRNA Therapeutics, Inc., Immagene, B.V. and Autoimmunity Biologic Solutions, Inc. J.R.C.-R. holds patents on the targeting of ER stress responses for the treatment of disease. S.C.-C.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks B. Tirosh, J. E. Thaxton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Conza, G., Ho, PC., Cubillos-Ruiz, J.R. et al. Control of immune cell function by the unfolded protein response. Nat Rev Immunol 23, 546–562 (2023). https://doi.org/10.1038/s41577-023-00838-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00838-0
This article is cited by
-
Endoplasmic reticulum stress and the unfolded protein response: emerging regulators in progression of traumatic brain injury
Cell Death & Disease (2024)
-
Non-mutational neoantigens in disease
Nature Immunology (2024)
-
Mammalian integrated stress responses in stressed organelles and their functions
Acta Pharmacologica Sinica (2024)