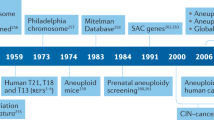
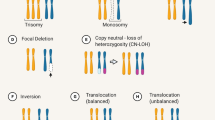
Abstract
Mosaic variegated aneuploidy (MVA) is a rare condition in which abnormal chromosome counts (that is, aneuploidies), affecting different chromosomes in each cell (making it variegated) are found only in a certain number of cells (making it mosaic). MVA is characterized by various developmental defects and, despite its rarity, presents a unique clinical scenario to understand the consequences of chromosomal instability and copy number variation in humans. Research from patients with MVA, genetically engineered mouse models and functional cellular studies have found the genetic causes to be mutations in components of the spindle-assembly checkpoint as well as in related proteins involved in centrosome dynamics during mitosis. MVA is accompanied by tumour susceptibility (depending on the genetic basis) as well as cellular and systemic stress, including chronic immune response and the associated clinical implications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nagaoka, S. I., Hassold, T. J. & Hunt, P. A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 (2012).
Liu, J. et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol. Reprod. 87, 148 (2012).
McCoy, R. C. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 33, 448–463 (2017).
Taylor, T. H. et al. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum. Reprod. Update 20, 571–581 (2014).
Zellweger, H. & Abbo, G. Familial mosaicism attributable to a new gene. Lancet 1, 455–457 (1965).
Tolmie, J. L. et al. Siblings with chromosome mosaicism, microcephaly, and growth retardation: the phenotypic expression of a human mitotic mutant? Hum. Genet. 80, 197–200 (1988).
Warburton, D., Anyane-Yeboa, K., Taterka, P., Yu, C. Y. & Olsen, D. Mosaic variegated aneuploidy with microcephaly: a new human mitotic mutant? Ann. Genet. 34, 287–292 (1991).
Kajii, T. et al. Cancer-prone syndrome of mosaic variegated aneuploidy and total premature chromatid separation: report of five infants. Am. J. Med. Genet. 104, 57–64 (2001).
Jacquemont, S., Boceno, M., Rival, J. M., Mechinaud, F. & David, A. High risk of malignancy in mosaic variegated aneuploidy syndrome. Am. J. Med. Genet. 109, 17–21 (2002).
Grange, L. J. et al. Pathogenic variants in SLF2 and SMC5 cause segmented chromosomes and mosaic variegated hyperploidy. Nat. Commun. 13, 6664 (2022).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004). This study reports biallelic mutations in the BUB1B gene in five patients with MVA syndrome, establishing for the first time a link between MVA syndrome and loss-of-function mutations in a core component of the SAC.
Snape, K. et al. Mutations in CEP57 cause mosaic variegated aneuploidy syndrome. Nat. Genet. 43, 527–529 (2011).
de Voer, R. M. et al. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are risk factors for colorectal cancer. Gastroenterology 145, 544–547 (2013).
Carvalhal, S. et al. Biallelic BUB1 mutations cause microcephaly, developmental delay, and variable effects on cohesion and chromosome segregation. Sci. Adv. 8, eabk0114 (2022).
Yost, S. et al. Biallelic TRIP13 mutations predispose to Wilms tumor and chromosome missegregation. Nat. Genet. 49, 1148–1151 (2017).
de Wolf, B. et al. Chromosomal instability by mutations in the novel minor spliceosome component CENATAC. EMBO J. 40, e106536 (2021).
Villarroya-Beltri, C. et al. Biallelic germline mutations in MAD1L1 induce a syndrome of aneuploidy with high tumor susceptibility. Sci. Adv. 8, eabq5914 (2022). This study uses single-cell transcriptomics to describe clonal selection and chronic immune response in a patient with MVA with an unprecedented number of tumours.
Abdel-Salam, G. M. H. et al. Biallelic MAD2L1BP (p31comet) mutation is associated with mosaic aneuploidy and juvenile granulosa cell tumors. JCI Insight 8, e170079 (2023).
Guo, J. et al. Mosaic variegated aneuploidy syndrome with tetraploid, and predisposition to male infertility triggered by mutant CEP192. HGG Adv. 5, 100256 (2024).
McAinsh, A. D. & Kops, G. Principles and dynamics of spindle assembly checkpoint signalling. Nat. Rev. Mol. Cell Biol. 24, 543–559 (2023).
Ricke, R. M. & van Deursen, J. M. Correction of microtubule-kinetochore attachment errors: mechanisms and role in tumor suppression. Semin. Cell Dev. Biol. 22, 559–565 (2011).
Matsuura, S. et al. Monoallelic BUB1B mutations and defective mitotic-spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome. Am. J. Med. Genet. A 140, 358–367 (2006).
Suijkerbuijk, S. J. et al. Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer Res. 70, 4891–4900 (2010).
Eytan, E. et al. Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31(comet). Proc. Natl Acad. Sci. USA 111, 12019–12024 (2014).
Ye, Q. et al. TRIP13 is a protein-remodeling AAA+ ATPase that catalyzes MAD2 conformation switching. eLife 4, e07367 (2015).
Alfieri, C., Chang, L. & Barford, D. Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13. Nature 559, 274–278 (2018).
Ma, H. T. & Poon, R. Y. C. TRIP13 regulates both the activation and inactivation of the spindle-assembly checkpoint. Cell Rep. 14, 1086–1099 (2016).
Hoang, D., Sue, G. R., Xu, F., Li, P. & Narayan, D. Absence of aneuploidy and gastrointestinal tumours in a man with a chromosomal 2q13 deletion and BUB1 monoallelic deficiency. BMJ Case Rep. 2013, bcr2013008684 (2013).
Mur, P. et al. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are infrequent in familial colorectal cancer and polyposis. Mol. Cancer 17, 23 (2018).
Fujita, H. et al. Premature aging syndrome showing random chromosome number instabilities with CDC20 mutation. Aging Cell 19, e13251 (2020). This study reports a unique case of MVA and premature ageing in a patient with a heterozygous germline missense mutation in CDC20.
Chen, O. J. et al. Germline missense variants in CDC20 result in aberrant mitotic progression and familial cancer. Cancer Res. 82, 3499–3515 (2022).
Villarroya-Beltri, C. & Malumbres, M. Mitotic checkpoint imbalances in familial cancer. Cancer Res. 82, 3432–3434 (2022).
Watanabe, K., Takao, D., Ito, K. K., Takahashi, M. & Kitagawa, D. The Cep57-pericentrin module organizes PCM expansion and centriole engagement. Nat. Commun. 10, 931 (2019).
Zhou, H., Wang, T., Zheng, T., Teng, J. & Chen, J. Cep57 is a Mis12-interacting kinetochore protein involved in kinetochore targeting of Mad1-Mad2. Nat. Commun. 7, 10151 (2016). This paper reports an unexpected function of CEP57 in the recruitment of MAD1 to kinetochores.
Bossard, C. et al. Translokin is an intracellular mediator of FGF-2 trafficking. Nat. Cell Biol. 5, 433–439 (2003).
Chinen, T. et al. Centriole and PCM cooperatively recruit CEP192 to spindle poles to promote bipolar spindle assembly. J. Cell Biol. 220, e202006085 (2021).
Park, J. G. et al. Structural basis for CEP192-mediated regulation of centrosomal AURKA. Sci. Adv. 9, eadf8582 (2023).
Bai, R. et al. Structural basis of U12-type intron engagement by the fully assembled human minor spliceosome. Science 383, 1245–1252 (2024).
Valcarcel, J. & Malumbres, M. Splicing together sister chromatids. EMBO J. 33, 2601–2603 (2014).
Wang, T., Zou, Y., Huang, N., Teng, J. & Chen, J. CCDC84 acetylation oscillation regulates centrosome duplication by modulating HsSAS-6 degradation. Cell Rep. 29, 2078–2091.e5 (2019).
Krivega, M., Stiefel, C. M. & Storchova, Z. Consequences of chromosome gain: a new view on trisomy syndromes. Am. J. Hum. Genet. 109, 2126–2140 (2022).
Torres, E. M. Consequences of gaining an extra chromosome. Chromosome Res. 31, 24 (2023).
Li, R. & Zhu, J. Effects of aneuploidy on cell behaviour and function. Nat. Rev. Mol. Cell Biol. 23, 250–265 (2022).
Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 8, 608 (2012).
Chunduri, N. K. & Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 21, 54–62 (2019).
Bolton, H. et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat. Commun. 7, 11165 (2016).
Pfau, S. J., Silberman, R. E., Knouse, K. A. & Amon, A. Aneuploidy impairs hematopoietic stem cell fitness and is selected against in regenerating tissues in vivo. Genes Dev. 30, 1395–1408 (2016).
Singla, S., Iwamoto-Stohl, L. K., Zhu, M. & Zernicka-Goetz, M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat. Commun. 11, 2958 (2020).
Pavelka, N. et al. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 468, 321–325 (2010).
Yona, A. H. et al. Chromosomal duplication is a transient evolutionary solution to stress. Proc. Natl Acad. Sci. USA 109, 21010–21015 (2012).
Watson, E. V. et al. Chromosome evolution screens recapitulate tissue-specific tumor aneuploidy patterns. Nat. Genet. 56, 900–912 (2024).
Schvartzman, J. M., Sotillo, R. & Benezra, R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer 10, 102–115 (2010).
Naylor, R. M. & van Deursen, J. M. Aneuploidy in cancer and aging. Annu. Rev. Genet. 50, 45–66 (2016).
Wang, Q. et al. BUBR1 deficiency results in abnormal megakaryopoiesis. Blood 103, 1278–1285 (2004).
Dai, W. et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 64, 440–445 (2004).
Simmons, A. J. et al. Nearly complete deletion of BubR1 causes microcephaly through shortened mitosis and massive cell death. Hum. Mol. Genet. 28, 1822–1836 (2019).
Baker, D. J. et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744–749 (2004). This pioneering study shows that BUBR1 is haploinsufficient and mutant mice expressing low levels of BUBR1 develop aneuploidy, infertility and progeroid features.
Baker, D. J. et al. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J. Cell Biol. 172, 529–540 (2006).
Sieben, C. J. et al. BubR1 allelic effects drive phenotypic heterogeneity in mosaic-variegated aneuploidy progeria syndrome. J. Clin. Invest. 130, 6188 (2020).
Baker, D. J. et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 15, 96–102 (2013). This paper describes a gain-of-function mouse model with overexpressesion of BUBR1. Sustained expression of BUBR1 reduces tumorigenesis, extends lifespan, and delays age-related deterioration and aneuploidy.
Weaver, R. L. et al. BubR1 alterations that reinforce mitotic surveillance act against aneuploidy and cancer. eLife 5, e16620 (2016).
Tilston, V., Taylor, S. S. & Perera, D. Inactivating the spindle checkpoint kinase Bub1 during embryonic development results in a global shutdown of proliferation. BMC Res. Notes 2, 190 (2009).
Schliekelman, M. et al. Impaired Bub1 function in vivo compromises tension-dependent checkpoint function leading to aneuploidy and tumorigenesis. Cancer Res. 69, 45–54 (2009).
Abdel-Hafiz, H. A. et al. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature 619, 624–631 (2023). This work shows that loss of the Y chromosome, the most common spontaneous aneuploidy, does not change proliferation in vitro but promotes exhaustion of CD8+ T cells in the tumour microenvironment and correlates with worse prognoses.
Wang, R. W., Vigano, S., Ben-David, U., Amon, A. & Santaguida, S. Aneuploid senescent cells activate NF-κB to promote their immune clearance by NK cells. EMBO Rep. 22, e52032 (2021).
Xian, S. et al. The unfolded protein response links tumor aneuploidy to local immune dysregulation. EMBO Rep. 22, e52509 (2021).
Kuang, X. & Li, J. Chromosome instability and aneuploidy as context-dependent activators or inhibitors of antitumor immunity. Front. Immunol. 13, 895961 (2022).
Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 41, 638–651.e5 (2017).
Senovilla, L. et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 337, 1678–1684 (2012).
Davoli, T., Uno, H., Wooten, E. C. & Elledge, S. J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355, eaaf8399 (2017). This study shows that tumour aneuploidy inversely correlates with markers of cytotoxic immune cells, especially CD8+ T cells, and predicts poor survival in patients undergoing immunotherapy.
van Jaarsveld, R. H. & Kops, G. Difference makers: chromosomal instability versus aneuploidy in cancer. Trends Cancer 2, 561–571 (2016).
Shoshani, O. et al. Transient genomic instability drives tumorigenesis through accelerated clonal evolution. Genes Dev. 35, 1093–1108 (2021).
Trakala, M. et al. Clonal selection of stable aneuploidies in progenitor cells drives high-prevalence tumorigenesis. Genes Dev. 35, 1079–1092 (2021).
Baker, D. J., Jin, F., Jeganathan, K. B. & van Deursen, J. M. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 16, 475–486 (2009).
Sotillo, R., Schvartzman, J. M., Socci, N. D. & Benezra, R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 464, 436–440 (2010).
Lukow, D. A. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 56, 2427–2439.e4 (2021).
Ippolito, M. R. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 56, 2440–2454.e6 (2021).
Replogle, J. M. et al. Aneuploidy increases resistance to chemotherapeutics by antagonizing cell division. Proc. Natl Acad. Sci. USA 117, 30566–30576 (2020). Refs. 75–78 provide elegant examples of how CIN contributes to improved fitness and response to stress conditions (therapeutic treatment) in tumour cells.
Rio Frio, T. et al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. N. Engl. J. Med. 363, 2628–2637 (2010).
Forsberg, L. A. et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat. Genet. 46, 624–628 (2014).
Ogawa, H., Horitani, K., Izumiya, Y. & Sano, S. Somatic mosaicism in biology and disease. Annu. Rev. Physiol. 84, 113–133 (2022).
Wang, Y. & Sano, S. Why Y matters? The implication of loss of Y chromosome in blood and cancer. Cancer Sci. 115, 706–714 (2024).
Wright, D. J. et al. Genetic variants associated with mosaic Y chromosome loss highlight cell cycle genes and overlap with cancer susceptibility. Nat. Genet. 49, 674–679 (2017). This study shows that allelic variants in SAC genes may contribute to loss of other chromosomes and associated pathologies.
Thompson, D. J. et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575, 652–657 (2019).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Hahn, M. M. et al. Prevalence of germline mutations in the spindle assembly checkpoint gene BUB1B in individuals with early-onset colorectal cancer. Genes Chromosomes Cancer 55, 855–863 (2016).
Tsukasaki, K. et al. Mutations in the mitotic check point gene, MAD1L1, in human cancers. Oncogene 20, 3301–3305 (2001).
Terao, C. et al. Chromosomal alterations among age-related haematopoietic clones in Japan. Nature 584, 130–135 (2020).
Zhong, R. et al. MAD1L1 Arg558His and MAD2L1 Leu84Met interaction with smoking increase the risk of colorectal cancer. Sci. Rep. 5, 12202 (2015).
Holland, A. J. & Cleveland, D. W. Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 13, 501–514 (2012).
Raaijmakers, J. A. et al. BUB1 is essential for the viability of human cells in which the spindle assembly checkpoint is compromised. Cell Rep. 22, 1424–1438 (2018).
Rodriguez-Rodriguez, J. A. et al. Distinct roles of RZZ and Bub1-KNL1 in mitotic checkpoint signaling and kinetochore expansion. Curr. Biol. 28, 3422–3429.e5 (2018).
Vanneste, E. et al. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 15, 577–583 (2009). A surprising analysis describing the commonality of chromosomal instability in postzygotic human embryos.
McCoy, R. C. et al. Evidence of selection against complex mitotic-origin aneuploidy during preimplantation development. PLoS Genet. 11, e1005601 (2015).
Sansregret, L. & Swanton, C. The role of aneuploidy in cancer evolution. Cold Spring Harb. Perspect. Med. 7, a028373 (2017).
Zhang, G. et al. Efficient mitotic checkpoint signaling depends on integrated activities of Bub1 and the RZZ complex. EMBO J. 38, e100977 (2019).
Wolthuis, R. et al. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell 30, 290–302 (2008).
Tsang, M. J. & Cheeseman, I. M. Alternative CDC20 translational isoforms tune mitotic arrest duration. Nature 617, 154–161 (2023).
Sansregret, L. et al. APC/C dysfunction limits excessive cancer chromosomal instability. Cancer Discov. 7, 218–233 (2017).
Ochiai, H. et al. TALEN-mediated single-base-pair editing identification of an intergenic mutation upstream of BUB1B as causative of PCS (MVA) syndrome. Proc. Natl Acad. Sci. USA 111, 1461–1466 (2014).
Kato, M. et al. PCS/MVA syndrome caused by an Alu insertion in the BUB1B gene. Hum. Genome Var. 4, 17021 (2017).
Laberko, A. et al. Hematopoietic stem cell transplantation in a patient with type 1 mosaic variegated aneuploidy syndrome. Orphanet J. Rare Dis. 14, 97 (2019).
Lin, S. M. et al. Prenatal diagnosis and long-term follow-up of a Chinese patient with mosaic variegated aneuploidy and its molecular analysis. Clin. Case Rep. 8, 1369–1375 (2020).
Pavone, P. et al. Pathogenic correlation between mosaic variegated aneuploidy 1 (MVA1) and a novel BUB1B variant: a reappraisal of a severe syndrome. Neurol. Sci. 43, 6529–6538 (2022).
He, T., Cui, D., Huang, Y., Luo, X. & Yang, J. Clinical features and genetic analysis of a child with mosaic variegated aneuploidy syndrome [Chinese]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 35, 844–847 (2018).
Pinson, L. et al. CEP57 mutation in a girl with mosaic variegated aneuploidy syndrome. Am. J. Med. Genet. A 164A, 177–181 (2014).
Santos-Simarro, F. et al. Mosaic variegated aneuploidy syndrome 2 caused by biallelic variants in CEP57, two new cases and review of the phenotype. Eur. J. Med. Genet. 64, 104338 (2021).
Feng, B. et al. A novel CEP57 variant associated with mosaic variegated aneuploidy syndrome in a Chinese female presenting with short stature, microcephaly, brachydactyly, and small teeth. Mol. Genet. Genom. Med. 10, e1951 (2022).
Hubner, C. T., Amin, A. K., Dey, D., Meyer, R. & Eggermann, T. Mosaic variegated aneuploidy syndrome and Noonan syndrome in the same family. Mol. Syndromol. 13, 402–408 (2022).
Langeh, N. et al. Mosaic variegated aneuploidy syndrome 2 with biallelic novel CEP57 splice site variation in Indian siblings: expanding the clinical and molecular spectrum. Clin. Genet. 103, 478–483 (2023).
De la Torre-Garcia, O. et al. A homozygous CEP57 c.915_925dupCAATGTTCAGC mutation in a patient with mosaic variegated aneuploidy syndrome with rhizomelic shortening in the upper and lower limbs and a narrow thorax. Eur. J. Med. Genet. 62, 195–197 (2019).
Brightman, D. S., Ejaz, S. & Dauber, A. Mosaic variegated aneuploidy syndrome caused by a CEP57 mutation diagnosed by whole exome sequencing. Clin. Case Rep. 6, 1531–1534 (2018).
Pezzani, L. et al. Double homozygosity in CEP57 and DYNC2H1 genes detected by WES: composite or expanded phenotype? Mol. Genet. Genom. Med. 8, e1064 (2020).
Dery, T. et al. Follow-up of two adult brothers with homozygous CEP57 pathogenic variants expands the phenotype of mosaic variegated aneuploidy syndrome. Eur. J. Med. Genet. 63, 104044 (2020).
Wijshake, T. et al. Reduced life- and healthspan in mice carrying a mono-allelic BubR1 MVA mutation. PLoS Genet. 8, e1003138 (2012).
Aziz, K. et al. Mosaic-variegated aneuploidy syndrome mutation or haploinsufficiency in Cep57 impairs tumor suppression. J. Clin. Invest. 128, 3517–3534 (2018).
Leland, S. et al. Heterozygosity for a Bub1 mutation causes female-specific germ cell aneuploidy in mice. Proc. Natl Acad. Sci. USA 106, 12776–12781 (2009).
Ricke, R. M., Jeganathan, K. B., Malureanu, L., Harrison, A. M. & van Deursen, J. M. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J. Cell Biol. 199, 931–949 (2012).
Li, X. C. & Schimenti, J. C. Mouse pachytene checkpoint 2 (trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 3, e130 (2007).
Iwanaga, Y. et al. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 67, 160–166 (2007).
Choi, E., Zhang, X., Xing, C. & Yu, H. Mitotic checkpoint regulators control insulin signaling and metabolic homeostasis. Cell 166, 567–581 (2016). This study demonstrates an unexpected functional connection between the MAD2–BUBR1–p31Comet axis and signalling by the insulin receptor.
Skibbens, R. V. et al. Cohesinopathies of a feather flock together. PLoS Genet. 9, e1004036 (2013).
Piche, J., Van Vliet, P. P., Puceat, M. & Andelfinger, G. The expanding phenotypes of cohesinopathies: one ring to rule them all! Cell Cycle 18, 2828–2848 (2019).
Davidson, I. F. & Peters, J. M. Genome folding through loop extrusion by SMC complexes. Nat. Rev. Mol. Cell Biol. 22, 445–464 (2021).
Losada, A. Cohesin in cancer: chromosome segregation and beyond. Nat. Rev. Cancer 14, 389–393 (2014).
Keijzers, G., Bakula, D. & Scheibye-Knudsen, M. Monogenic diseases of DNA repair. N. Engl. J. Med. 377, 1868–1876 (2017).
Jayaraman, D., Bae, B. I. & Walsh, C. A. The genetics of primary microcephaly. Annu. Rev. Genomics Hum. Genet. 19, 177–200 (2018).
Yamashita, D. et al. MCPH1 regulates chromosome condensation and shaping as a composite modulator of condensin II. J. Cell Biol. 194, 841–854 (2011).
Hoencamp, C. & Rowland, B. D. Genome control by SMC complexes. Nat. Rev. Mol. Cell Biol. 24, 633–650 (2023).
Vader, G. Pch2(TRIP13): controlling cell division through regulation of HORMA domains. Chromosoma 124, 333–339 (2015).
Bhalla, N. PCH-2 and meiotic HORMADs: a module for evolutionary innovation in meiosis? Curr. Top. Dev. Biol. 151, 317–344 (2023).
Zhang, Z. et al. Bi-allelic missense pathogenic variants in TRIP13 cause female infertility characterized by oocyte maturation arrest. Am. J. Hum. Genet. 107, 15–23 (2020).
Huang, L. et al. Biallelic variants in MAD2L1BP (p31(comet)) cause female infertility characterized by oocyte maturation arrest. eLife 12, e85649 (2023).
Clairmont, C. S. et al. TRIP13 regulates DNA repair pathway choice through REV7 conformational change. Nat. Cell Biol. 22, 87–96 (2020).
Sarangi, P., Clairmont, C. S., Galli, L. D., Moreau, L. A. & D’Andrea, A. D. p31(comet) promotes homologous recombination by inactivating REV7 through the TRIP13 ATPase. Proc. Natl Acad. Sci. USA 117, 26795–26803 (2020).
de Krijger, I. et al. MAD2L2 dimerization and TRIP13 control shieldin activity in DNA repair. Nat. Commun. 12, 5421 (2021).
Jeong, H. et al. TRIP13 participates in immediate-early sensing of DNA strand breaks and ATM signaling amplification through MRE11. Cells 11, 4095 (2022).
Li, F. et al. The BUB3-BUB1 complex promotes telomere DNA replication. Mol. Cell 70, 395–407.e4 (2018).
Xiao, M. et al. Kinetochore protein MAD1 participates in the DNA damage response through ataxia-telangiectasia mutated kinase-mediated phosphorylation and enhanced interaction with KU80. Cancer Biol. Med. 17, 640–651 (2020).
Jessulat, M. et al. Spindle checkpoint factors Bub1 and Bub2 promote DNA double-strand break repair by nonhomologous end joining. Mol. Cell Biol. 35, 2448–2463 (2015).
Miyamoto, T. et al. Insufficiency of BUBR1, a mitotic spindle checkpoint regulator, causes impaired ciliogenesis in vertebrates. Hum. Mol. Genet. 20, 2058–2070 (2011).
Baker, D. J. et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 10, 825–836 (2008).
Choi, E. & Yu, H. Spindle checkpoint regulators in insulin signaling. Front. Cell Dev. Biol. 6, 161 (2018).
Yang, S. et al. Bub1 facilitates virus entry through endocytosis in a model of Drosophila pathogenesis. J. Virol. 92, e00254-18 (2018).
Nyati, S., Young, G., Speers, C., Nyati, M. K. & Rehemtulla, A. Budding uninhibited by benzimidazoles-1 (BUB1) regulates EGFR signaling by reducing EGFR internalization. Aging 15, 6011–6030 (2023).
Nyati, S. et al. The kinase activity of the Ser/Thr kinase BUB1 promotes TGF-β signaling. Sci. Signal. 8, ra1 (2015).
Yoshida, S. et al. Bub1 suppresses inflammatory arthritis-associated bone loss in mice through inhibition of TNFα-mediated osteoclastogenesis. J. Bone Min. Res. 39, 341–356 (2024).
Zhang, Q. et al. Bub1 and Bub3 regulate metabolic adaptation via macrolipophagy in Drosophila. Cell Rep. 42, 112343 (2023).
Wan, J. et al. A Golgi-localized pool of the mitotic checkpoint component Mad1 controls integrin secretion and cell migration. Curr. Biol. 24, 2687–2692 (2014).
Goo, B. S. et al. Schizophrenia-associated mitotic arrest deficient-1 (MAD1) regulates the polarity of migrating neurons in the developing neocortex. Mol. Psychiatry 28, 856–870 (2023).
Wan, J. et al. Mad1 destabilizes p53 by preventing PML from sequestering MDM2. Nat. Commun. 10, 1540 (2019).
Acknowledgements
We thank Adrià López Fernández and Judith Balmaña (VHIO) and Ana Losada (CNIO, Madrid) for helpful discussions and comments on the manuscript. C.V.-B. received salary support from Amigos del CNIO, Madrid, and the Ramon y Cajal programme from the Ministerio de Ciencia y Universidades (MICIU)-Agencia Estatal de Investigación (AEI) (RYC2022-035259-I). The M.M. laboratory is supported by research grants from MICIU-AEI/FEDER (PID2021-128726, PDC2022-133408-I00 and RED2022-134792-T), Comunidad de Madrid (Y2020/BIO-6519 and S2022/BMD-7437), Asociación Española Contra el Cáncer (AECC) and the José Baselga Innovative Disruption programme from AstraZeneca. VHIO (CEX2020-001024-S/AEI/10.13039/501100011033) and CNIO (CEX2019-000891-S) are Centers of Excellence Severo Ochoa (MICIU-AEI).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Geert Kops and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
cBioPortal: https://www.cbioportal.org/
COSMIC: https://cancer.sanger.ac.uk/cosmic
Genomic Data Commons Data Portal: https://portal.gdc.cancer.gov/
Mitelman Database: https://mitelmandatabase.isb-cgc.org/
OMIM: https://www.omim.org/
Glossary
- Autosomal recessive
-
A mode of inheritance in which the symptoms of the disease only manifest in individuals with syndrome-causing mutations in both alleles of the gene (one inherited from the mother and the other from the father).
- Cell-cycle checkpoints
-
A signalling pathway ensuring that the cell has properly terminated the previous cell-cycle phase before transitioning to the next phase.
- Chromosomal instability
-
(CIN). The acquired elevated chance of unequal distribution of genomic content between two daughter cells and is thus a ‘cellular phenotype’. The level of CIN refers to the frequency and severity of these errors over successive cell-division cycles.
- Genome instability
-
An increased tendency to acquire alterations in the genome, ranging from single nucleotide modifications to structural or numerical alterations of whole chromosomes.
- Hypomorphic
-
A type of mutation that causes only partial loss of function of the protein encoded.
- Lordokyphosis
-
A condition characterized by a combination of lordosis and kyphosis, where the spine exhibits abnormal curvature both inward (lordosis) and outward (kyphosis) simultaneously.
- Mitotic checkpoint complex
-
(MCC). The network of proteins responsible for inhibiting the ubiquitin ligase activity of the anaphase-promoting complex/cyclosome in the spindle-assembly checkpoint. It maintains cells in prometaphase until all chromosomes are properly attached to the mitotic spindle.
- Monosomy
-
A form of aneuploidy in which only one chromosome from a pair of homologous chromosomes is present.
- Tetraploidy
-
The condition of having four, instead of two, complete sets of chromosomes.
- Trisomy
-
A form of aneuploidy in which homologous chromosomes are represented by three copies instead of two.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malumbres, M., Villarroya-Beltri, C. Mosaic variegated aneuploidy in development, ageing and cancer. Nat Rev Genet (2024). https://doi.org/10.1038/s41576-024-00762-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41576-024-00762-6