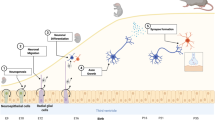
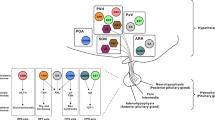
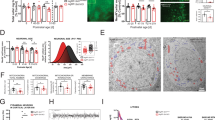
Abstract
The tuberal hypothalamus regulates a range of crucial physiological processes, including energy homeostasis and metabolism. In this Review, we explore the intricate molecular mechanisms and signalling pathways that control the development of the tuberal hypothalamus, focusing on aspects that shape metabolic outcomes. Major developmental events are discussed in the context of their effect on the establishment of both functional hypothalamic neuronal circuits and brain–body interfaces that are pivotal to the control of metabolism. Emerging evidence indicates that aberrations in molecular pathways during tuberal hypothalamic development contribute to metabolic dysregulation. Understanding the molecular underpinnings of tuberal hypothalamic development provides a comprehensive view of neurodevelopmental processes and offers a promising avenue for future targeted interventions to prevent and treat metabolic disorders.
Key points
-
Energy balance is regulated by pro-opiomelanocortin and neuropeptide Y neurons within the arcuate nucleus and by tanycytes within the median eminence.
-
Sequential signalling events govern the progression of diencephalic prethalamic-like cells to generate regionally distinct populations of hypothalamic progenitors; sustained signalling events and hierarchical transcription factor networks mediate tuberal neurogenesis and the specification of tuberal neuronal subtypes and tanycytes.
-
Leptin and a high-fat diet regulate diverse aspects of tuberal cell specification, including neurogenesis, axon guidance and synaptic connectivity.
-
Neurons of the tuberal hypothalamus are sexually dimorphic in their distribution and regulate sexually dimorphic patterns of behaviour.
-
Genetic and environmental factors disrupt tuberal hypothalamic development and lead to lifelong metabolic defects.
-
Directed differentiation of human induced pluripotent stem cells towards hypothalamic identities is in its infancy but holds the promise of generating therapeutically important tuberal hypothalamic cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tessmar-Raible, K. et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129, 1389–1400 (2007).
Fong, H., Zheng, J. & Kurrasch, D. The structural and functional complexity of the integrative hypothalamus. Science 382, 388–394 (2023).
Alcantara, I. C., Tapia, A. P. M., Aponte, Y. & Krashes, M. J. Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat. Metab. 4, 836–847 (2022).
Wang, D. et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 9, 40 (2015).
Dietrich, M. O. et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat. Neurosci. 15, 1108–1110 (2012).
Prevot, V. et al. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: functional consequences for reproduction and dynamic role of vascular endothelial cells. J. Neuroendocrinol. 22, 639–649 (2010).
García-Cáceres, C. et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 22, 7–14 (2019).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667.e6 (2019).
Schaeffer, M. et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl Acad. Sci. USA 110, 1512–1517 (2013).
Balland, E. et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301 (2014).
Yoo, S. et al. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia 68, 1987–2000 (2020).
Yoo, S., Cha, D., Kim, D. W., Hoang, T. V. & Blackshaw, S. Tanycyte-independent control of hypothalamic leptin signaling. Front. Neurosci. 13, 240 (2019).
Lee, D. A. et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 15, 700–702 (2012).
Robins, S. C. et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 (2013).
Theodosis, D. T., Poulain, D. A. & Oliet, S. H. R. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 88, 983–1008 (2008).
Anbalagan, S. et al. Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Dev. Cell 47, 711–726.e5 (2018).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Chen, R., Wu, X., Jiang, L. & Zhang, Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 18, 3227–3241 (2017).
Kim, D. W. et al. The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat. Commun. 11, 4360 (2020).
Romanov, R. A. et al. Molecular design of hypothalamus development. Nature 582, 246–252 (2020).
Mickelsen, L. E. et al. Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. eLife 9, e58901 (2020).
Ma, T., Wong, S. Z. H., Lee, B., Ming, G.-L. & Song, H. Decoding neuronal composition and ontogeny of individual hypothalamic nuclei. Neuron 109, 1150–1167.e6 (2021).
Steuernagel, L. et al. HypoMap-a unified single-cell gene expression atlas of the murine hypothalamus. Nat. Metab. 4, 1402–1419 (2022).
Yao, Z. et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624, 317–332 (2023).
Harkany, T. et al. Molecularly stratified hypothalamic astrocytes are cellular foci for obesity. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-3748581/v1 (2024).
Sullivan, A. I., Potthoff, M. J. & Flippo, K. H. Tany-seq: integrated analysis of the mouse tanycyte transcriptome. Cells 11, 1565 (2022).
Placzek, M. & Briscoe, J. The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230–240 (2005).
Kim, D. W. et al. Single-cell analysis of early chick hypothalamic development reveals that hypothalamic cells are induced from prethalamic-like progenitors. Cell Rep. 38, 110251 (2022).
Chinnaiya, K. et al. A neuroepithelial wave of BMP signalling drives anteroposterior specification of the tuberal hypothalamus. eLife 12, e83133 (2023).
Manning, L. et al. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell 11, 873–885 (2006).
Dale, J. K. et al. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90, 257–269 (1997).
Dale, K. et al. Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin. Development 126, 397–408 (1999).
Fu, T., Towers, M. & Placzek, M. A. Fgf10+ progenitors give rise to the chick hypothalamus by rostral and caudal growth and differentiation. Development 144, 3278–3288 (2017).
Szabó, N.-E. et al. Role of neuroepithelial sonic hedgehog in hypothalamic patterning. J. Neurosci. 29, 6989–7002 (2009).
Shimogori, T. et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 13, 767–775 (2010).
Haddad-Tóvolli, R. et al. Differential requirements for Gli2 and Gli3 in the regional specification of the mouse hypothalamus. Front. Neuroanat. 9, 34 (2015).
Puelles, L. Recollections on the origins and development of the prosomeric model. Front. Neuroanat. 15, 787913 (2021).
Alvarez-Bolado, G., Grinevich, V. & Puelles, L. Editorial: development of the hypothalamus. Front. Neuroanat. 9, 83 (2015).
Manning, E. & Placzek, M. Organizing activities of axial mesoderm. Curr. Top. Dev. Biol. 157, 83–123 (2024).
Aoto, K. et al. Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev. Biol. 327, 106–120 (2009).
Placzek, M. & Briscoe, J. Sonic hedgehog in vertebrate neural tube development. Int. J. Dev. Biol. 62, 225–234 (2018).
Barratt, K. S., Drover, K. A., Thomas, Z. M. & Arkell, R. M. Patterning of the antero-ventral mammalian brain: lessons from holoprosencephaly comparative biology in man and mouse. WIREs Mech. Dis. 14, e1552 (2022).
Shimamura, K. & Rubenstein, J. L. Inductive interactions direct early regionalization of the mouse forebrain. Development 124, 2709–2718 (1997).
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996).
Mukhopadhyay, M. et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell 1, 423–434 (2001).
Lewis, S. L. et al. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development 135, 1791–1801 (2008).
Lagutin, O. V. et al. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 17, 368–379 (2003).
Newman, E. A., Wu, D., Taketo, M. M., Zhang, J. & Blackshaw, S. Canonical Wnt signaling regulates patterning, differentiation and nucleogenesis in mouse hypothalamus and prethalamus. Dev. Biol. 442, 236–248 (2018).
Geng, X. et al. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev. Cell 15, 236–247 (2008).
Muthu, V., Eachus, H., Ellis, P., Brown, S. & Placzek, M. Rx3 and Shh direct anisotropic growth and specification in the zebrafish tuberal/anterior hypothalamus. Development 143, 2651–2663 (2016).
Orquera, D. P., Nasif, S., Low, M. J., Rubinstein, M. & de Souza, F. S. J. Essential function of the transcription factor Rax in the early patterning of the mammalian hypothalamus. Dev. Biol. 416, 212–224 (2016).
Manoli, M. & Driever, W. nkx2.1 and nkx2.4 genes function partially redundant during development of the zebrafish hypothalamus, preoptic region, and pallidum. Front. Neuroanat. 8, 145 (2014).
Sussel, L., Marin, O., Kimura, S. & Rubenstein, J. L. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370 (1999).
Strähle, U., Blader, P. & Ingham, P. W. Expression of axial and sonic hedgehog in wildtype and midline defective zebrafish embryos. Int. J. Dev. Biol. 40, 929–940 (1996).
Mathieu, J., Barth, A., Rosa, F. M., Wilson, S. W. & Peyriéras, N. Distinct and cooperative roles for Nodal and Hedgehog signals during hypothalamic development. Development 129, 3055–3065 (2002).
Ellis, P. S. et al. ProNodal acts via FGFR3 to govern duration of Shh expression in the prechordal mesoderm. Development 142, 3821–3832 (2015).
Angueyra, J. M. et al. Transcription factors underlying photoreceptor diversity. eLife 12, e81579 (2023).
Pearson, C. A. et al. FGF-dependent midline-derived progenitor cells in hypothalamic infundibular development. Development 138, 2613–2624 (2011).
Corman, T. S., Bergendahl, S. E. & Epstein, D. J. Distinct temporal requirements for Sonic hedgehog signaling in development of the tuberal hypothalamus. Development 145, dev167379 (2018).
Carreno, G. et al. Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors. Development 144, 3289–3302 (2017).
Trowe, M.-O. et al. Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development 140, 2299–2309 (2013).
Goto, M. et al. Hes1 and Hes5 are required for differentiation of pituicytes and formation of the neurohypophysis in pituitary development. Brain Res. 1625, 206–217 (2015).
Salvatierra, J. et al. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J. Neurosci. 34, 16809–16820 (2014).
Oliver, G. et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045–4055 (1995).
Jean, D., Bernier, G. & Gruss, P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech. Dev. 84, 31–40 (1999).
Ikeda, Y., Luo, X., Abbud, R., Nilson, J. H. & Parker, K. L. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinol. 9, 478–486 (1995).
Fujiyama, T. et al. Forebrain Ptf1a is required for sexual differentiation of the brain. Cell Rep. 24, 79–94 (2018).
Wang, W., Grimmer, J. F., Van De Water, T. R. & Lufkin, T. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev. Cell 7, 439–453 (2004).
Pontecorvi, M., Goding, C. R., Richardson, W. D. & Kessaris, N. Expression of Tbx2 and Tbx3 in the developing hypothalamic-pituitary axis. Gene Expr. Patterns 8, 411–417 (2008).
Satoh, A., Brace, C. S., Rensing, N. & Imai, S.-I. Deficiency of Prdm13, a dorsomedial hypothalamus-enriched gene, mimics age-associated changes in sleep quality and adiposity. Aging Cell 14, 209–218 (2015).
Shi, X. et al. Hierarchical deployment of Tbx3 dictates the identity of hypothalamic KNDy neurons to control puberty onset. Sci. Adv. 8, eabq2987 (2022).
Shimada, M. & Nakamura, T. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 41, 163–173 (1973).
Lee, J. E., Wu, S.-F., Goering, L. M. & Dorsky, R. I. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development 133, 4451–4461 (2006).
Aujla, P. K., Naratadam, G. T., Xu, L. & Raetzman, L. T. Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons. Development 140, 3511–3521 (2013).
Biehl, M. J. & Raetzman, L. T. Rbpj-κ mediated Notch signaling plays a critical role in development of hypothalamic Kisspeptin neurons. Dev. Biol. 406, 235–246 (2015).
Ratié, L. et al. Novel genes upregulated when NOTCH signalling is disrupted during hypothalamic development. Neural Dev. 8, 25 (2013).
Ware, M., Hamdi-Rozé, H. & Dupé, V. Notch signaling and proneural genes work together to control the neural building blocks for the initial scaffold in the hypothalamus. Front. Neuroanat. 8, 140 (2014).
Place, E. et al. SHH and Notch regulate SOX9+ progenitors to govern arcuate POMC neurogenesis. Front. Neurosci. 16, 855288 (2022).
Pierfelice, T., Alberi, L. & Gaiano, N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840–855 (2011).
Engler, A., Zhang, R. & Taylor, V. Notch and neurogenesis. Adv. Exp. Med. Biol. 1066, 223–234 (2018).
Aslanpour, S., Han, S., Schuurmans, C. & Kurrasch, D. M. Acts as a classical proneural gene in the ventromedial hypothalamus and is required for the early phase of neurogenesis. J. Neurosci. 40, 3549–3563 (2020).
Pelling, M. et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 349, 406–416 (2011).
Hael, C. E., Rojo, D., Orquera, D. P., Low, M. J. & Rubinstein, M. The transcriptional regulator PRDM12 is critical for Pomc expression in the mouse hypothalamus and controlling food intake, adiposity, and body weight. Mol. Metab. 34, 43–53 (2020).
Lee, B. et al. Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat. Commun. 9, 2026 (2018).
Lee, B., Lee, S., Lee, S.-K. & Lee, J. W. The LIM-homeobox transcription factor Isl1 plays crucial roles in the development of multiple arcuate nucleus neurons. Development 143, 3763–3773 (2016).
Padilla, S. L., Carmody, J. S. & Zeltser, L. M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 16, 403–405 (2010).
Yu, H., Rubinstein, M. & Low, M. J. Developmental single-cell transcriptomics of hypothalamic POMC neurons reveal the genetic trajectories of multiple neuropeptidergic phenotypes. eLife 11, e72883 (2022).
Orquera, D. P. et al. The homeodomain transcription factor NKX2.1 is essential for the early specification of melanocortin neuron identity and activates expression in the developing hypothalamus. J. Neurosci. 39, 4023–4035 (2019).
Quarta, C. et al. Functional identity of hypothalamic melanocortin neurons depends on Tbx3. Nat. Metab. 1, 222–235 (2019).
Nasif, S. et al. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc. Natl Acad. Sci. USA 112, E1861–E1870 (2015).
Chen, X. et al. Comparative transcriptomic analyses of developing melanocortin neurons reveal new regulators for the anorexigenic neuron identity. J. Neurosci. 40, 3165–3177 (2020).
Jing, E., Nillni, E. A., Sanchez, V. C., Stuart, R. C. & Good, D. J. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides α melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology 145, 1503–1513 (2004).
Carraro, R. S. et al. Arcuate nucleus overexpression of NHLH2 reduces body mass and attenuates obesity-associated anxiety/depression-like behavior. J. Neurosci. 41, 10004–10022 (2021).
Croizier, S., Park, S., Maillard, J. & Bouret, S. G. Central Dicer-miR-103/107 controls developmental switch of POMC progenitors into NPY neurons and impacts glucose homeostasis. eLife 7, e40429 (2018).
Nogueiras, R. et al. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 149, 3009–3015 (2008).
Sokolowski, K. et al. Specification of select hypothalamic circuits and innate behaviors by the embryonic patterning gene dbx1. Neuron 86, 403–416 (2015).
Li, H., Zeitler, P. S., Valerius, M. T., Small, K. & Potter, S. S. Gsh-1, an orphan Hox gene, is required for normal pituitary development. EMBO J. 15, 714–724 (1996).
Huisman, C. et al. Single cell transcriptome analysis of developing arcuate nucleus neurons uncovers their key developmental regulators. Nat. Commun. 10, 3696 (2019).
Yee, C. L., Wang, Y., Anderson, S., Ekker, M. & Rubenstein, J. L. R. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y+, proopiomelanocortin+, and tyrosine hydroxylase+ neurons in neonatal and adult mice. J. Comp. Neurol. 517, 37–50 (2009).
Zhang, Q. et al. Satb2 regulates the development of dopaminergic neurons in the arcuate nucleus by Dlx1. Cell Death Dis. 12, 879 (2021).
Atkin, S. D. et al. Nuclear receptor LRH-1 induces the reproductive neuropeptide kisspeptin in the hypothalamus. Mol. Endocrinol. 27, 598–605 (2013).
Whittaker, D. E. et al. A recessive PRDM13 mutation results in congenital hypogonadotropic hypogonadism and cerebellar hypoplasia. J. Clin. Invest. 131, e141587 (2021).
Altman, J. & Bayer, S. A. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J. Comp. Neurol. 182, 995–1015 (1978).
Lopez-Rodriguez, D. et al. Ontogeny of ependymoglial cells lining the third ventricle in mice. Front. Endocrinol. 13, 1073759 (2022).
Yoo, S. & Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 170, 53–66 (2018).
Miranda-Angulo, A. L., Byerly, M. S., Mesa, J., Wang, H. & Blackshaw, S. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. J. Comp. Neurol. 522, 876–899 (2014).
Moore, A. et al. Loss of function of the neural cell adhesion molecule NrCAM regulates differentiation, proliferation and neurogenesis in early postnatal hypothalamic tanycytes. Front. Neurosci. 16, 832961 (2022).
Parkash, J. et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat. Commun. 6, 6385 (2015).
Yoo, S. et al. Control of neurogenic competence in mammalian hypothalamic tanycytes. Sci. Adv. 7, eabg3777 (2021).
Lee, D. A. et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front. Neurosci. 8, 157 (2014).
Goodman, T. et al. Fibroblast growth factor 10 is a negative regulator of postnatal neurogenesis in the mouse hypothalamus. Development 147, dev180950 (2020).
Recabal, A. et al. The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. J. Neurochem. 156, 182–199 (2021).
Son, J. E. et al. Irx3 and Irx5 in Ins2-Cre cells regulate hypothalamic postnatal neurogenesis and leptin response. Nat. Metab. 3, 701–713 (2021).
Dou, Z., Son, J. E. & Hui, C. C. Irx3 and Irx5 — novel regulatory factors of postnatal hypothalamic neurogenesis. Front. Neurosci. 15, 763856 (2021).
Makarenko, I. G., Ugrumov, M. V. & Calas, A. Axonal projections from the hypothalamus to the median eminence in rats during ontogenesis: diI tracing study. Anat. Embryol. 204, 239–252 (2001).
Liu, F. et al. Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development 140, 1111–1122 (2013).
Liu, F., Placzek, M. & Xu, H. Axon guidance effect of classical morphogens Shh and BMP7 in the hypothalamo-pituitary system. Neurosci. Lett. 553, 104–109 (2013).
Makarenko, I. G. DiI tracing of the hypothalamic projection systems during perinatal development. Front. Neuroanat. 8, 144 (2014).
Jais, A. & Brüning, J. C. Arcuate nucleus-dependent regulation of metabolism-pathways to obesity and diabetes mellitus. Endocr. Rev. 43, 314–328 (2022).
Gervais, M., Labouèbe, G., Picard, A., Thorens, B. & Croizier, S. EphrinB1 modulates glutamatergic inputs into POMC-expressing progenitors and controls glucose homeostasis. PLoS Biol. 18, e3000680 (2020).
Bouret, S. G., Draper, S. J. & Simerly, R. B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 24, 2797–2805 (2004).
van der Klaauw, A. A. et al. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell 176, 729–742.e18 (2019).
Coupé, B. et al. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 15, 247–255 (2012).
Lee, C. H. et al. Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat. Commun. 11, 5772 (2020).
Swithers, S. E. Do metabolic signals stimulate intake in rat pups? Physiol. Behav. 79, 71–78 (2003).
Steculorum, S. M. & Bouret, S. G. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 152, 4171–4179 (2011).
Bouret, S. G., Bates, S. H., Chen, S., Myers, M. G. Jr & Simerly, R. B. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 32, 1244–1252 (2012).
Kamitakahara, A., Bouyer, K., Wang, C.-H. & Simerly, R. A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J. Comp. Neurol. 526, 133–145 (2018).
Mirzadeh, Z. et al. Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat. Metab. 1, 212–221 (2019).
Nilsson, I., Johansen, J. E., Schalling, M., Hökfelt, T. & Fetissov, S. O. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res. Dev. Brain Res. 155, 147–154 (2005).
Bouret, S. G., Draper, S. J. & Simerly, R. B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004).
Freeman, G. M. et al. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron 78, 799–806 (2013).
Fan, J. et al. Vasoactive intestinal polypeptide (VIP)-expressing neurons in the suprachiasmatic nucleus provide sparse GABAergic outputs to local neurons with circadian regulation occurring distal to the opening of postsynaptic GABAA ionotropic receptors. J. Neurosci. 35, 1905–1920 (2015).
Shao, Y.-Q., Fan, L., Wu, W.-Y., Zhu, Y.-J. & Xu, H.-T. A developmental switch between electrical and neuropeptide communication in the ventromedial hypothalamus. Curr. Biol. 32, 3137–3145.e3 (2022).
Burdakov, D. & Karnani, M. M. Ultra-sparse connectivity within the lateral hypothalamus. Curr. Biol. 30, 4063–4070.e2 (2020).
Newton, A. J. et al. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology 154, 172–183 (2013).
Baquero, A. F. et al. Developmental changes in synaptic distribution in arcuate nucleus neurons. J. Neurosci. 35, 8558–8569 (2015).
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013).
Krause, W. C. et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599, 131–135 (2021).
Liu, M., Kim, D.-W., Zeng, H. & Anderson, D. J. Make war not love: the neural substrate underlying a state-dependent switch in female social behavior. Neuron 110, 841–856.e6 (2022).
McCarthy, M. M. et al. The epigenetics of sex differences in the brain. J. Neurosci. 29, 12815–12823 (2009).
Bell, M. R. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 159, 2596–2613 (2018).
Immenschuh, J. et al. Sex differences in distribution and identity of aromatase gene expressing cells in the young adult rat brain. Biol. Sex. Differ. 14, 54 (2023).
Shah, N. M. et al. Visualizing sexual dimorphism in the brain. Neuron 43, 313–319 (2004).
Knoedler, J. R. et al. A functional cellular framework for sex and estrous cycle-dependent gene expression and behavior. Cell 185, 654–671.e22 (2024).
Gegenhuber, B., Wu, M. V., Bronstein, R. & Tollkuhn, J. Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606, 153–159 (2022).
Griffin, G. D. & Flanagan-Cato, L. M. Ovarian hormone action in the hypothalamic ventromedial nucleus: remodelling to regulate reproduction. J. Neuroendocrinol. 23, 465–471 (2011).
Cabrera Zapata, L. E., Bollo, M. & Cambiasso, M. J. Estradiol-mediated axogenesis of hypothalamic neurons requires ERK1/2 and ryanodine receptors-dependent intracellular ca rise in male rats. Front. Cell. Neurosci. 13, 122 (2019).
Cisternas, C. D. et al. Estradiol-dependent axogenesis and Ngn3 expression are determined by XY sex chromosome complement in hypothalamic neurons. Sci. Rep. 10, 8223 (2020).
Tekendo-Ngongang, C., Muenke, M. & Kruszka, P. in GeneReviews® (eds Adam, M. P. et al.) https://www.ncbi.nlm.nih.gov/books/NBK1530/ (Univ. Washington, Seattle, 2000).
Diaz, C. & Puelles, L. Developmental genes and malformations in the hypothalamus. Front. Neuroanat. 14, 607111 (2020).
Angulo, M. A., Butler, M. G. & Cataletto, M. E. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J. Endocrinol. Invest. 38, 1249–1263 (2015).
Maillard, J. et al. Loss of Magel2 impairs the development of hypothalamic anorexigenic circuits. Hum. Mol. Genet. 25, 3208–3215 (2016).
Mercer, R. E. et al. Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet. 9, e1003207 (2013).
Anthwal, N. et al. Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity. Dis. Model. Mech. 6, 1133–1145 (2013).
Moir, L. et al. Disruption of the homeodomain transcription factor orthopedia homeobox (Otp) is associated with obesity and anxiety. Mol. Metab. 6, 1419–1428 (2017).
Farooqi, I. S. Monogenic obesity syndromes provide insights into the hypothalamic regulation of appetite and associated behaviors. Biol. Psychiatry 91, 856–859 (2022).
Loos, R. J. F. & Yeo, G. S. H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 23, 120–133 (2022).
Loid, P. et al. Rare variants in genes linked to appetite control and hypothalamic development in early-onset severe obesity. Front. Endocrinol. 11, 81 (2020).
Shugart, Y. Y. et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 17, 1050–1055 (2009).
Brewer, K. M., Brewer, K. K., Richardson, N. C. & Berbari, N. F. Neuronal cilia in energy homeostasis. Front. Cell Dev. Biol. 10, 1082141 (2022).
Guo, D.-F. et al. The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes 68, 1591–1603 (2019).
Guo, D.-F. et al. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet. 12, e1005890 (2016).
Woo Baidal, J. A. et al. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am. J. Prev. Med. 50, 761–779 (2016).
Larqué, E. et al. From conception to infancy - early risk factors for childhood obesity. Nat. Rev. Endocrinol. 15, 456–478 (2019).
Furigo, I. C. & Dearden, L. Mechanisms mediating the impact of maternal obesity on offspring hypothalamic development and later function. Front. Endocrinol. 13, 1078955 (2022).
Lemes, S. F. et al. Maternal consumption of high-fat diet in mice alters hypothalamic notch pathway, NPY cell population and food intake in offspring. Neuroscience 371, 1–15 (2018).
Dearden, L., Buller, S., Furigo, I. C., Fernandez-Twinn, D. S. & Ozanne, S. E. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol. Metab. 42, 101079 (2020).
Bae-Gartz, I. et al. Maternal obesity alters neurotrophin-associated MAPK signaling in the hypothalamus of male mouse offspring. Front. Neurosci. 13, 962 (2019).
Gali Ramamoorthy, T., Begum, G., Harno, E. & White, A. Developmental programming of hypothalamic neuronal circuits: impact on energy balance control. Front. Neurosci. 9, 126 (2015).
Catalano, P. M., Presley, L., Minium, J. & Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080 (2009).
Chandrasekaran, S. et al. Exposure to gestational diabetes mellitus prior to 26 weeks is related to the presence of mediobasal hypothalamic gliosis in children. Diabetes 71, 2552–2556 (2022).
Wataya, T. et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl Acad. Sci. USA 105, 11796–11801 (2008).
Wang, L. et al. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J. Clin. Invest. 125, 796–808 (2015).
Wang, L. et al. PC1/3 deficiency impacts pro-opiomelanocortin processing in human embryonic stem cell-derived hypothalamic neurons. Stem Cell Reports 8, 264–277 (2017).
Merkle, F. T. et al. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 142, 633–643 (2015).
Kano, M. et al. Tanycyte-like cells derived from mouse embryonic stem culture show hypothalamic neural stem/progenitor cell functions. Endocrinology 160, 1701–1718 (2019).
Miwata, T. et al. Generation of hypothalamic neural stem cell-like cells in vitro from human pluripotent stem cells. Stem Cell Reports 18, 869–883 (2023).
Kasai, T. et al. Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS cells. Cell Rep. 30, 18–24.e5 (2020).
Rajamani, U. et al. Super-obese patient-derived iPSc hypothalamic neurons exhibit obesogenic signatures and hormone responses. Cell Stem Cell 22, 698–712.e9 (2018).
Huang, W.-K. et al. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28, 1657–1670.e10 (2021).
Kowalko, J. Utilizing the blind cavefish to understand the genetic basis of behavioral evolution. J. Exp. Biol. 223, jeb208835 (2020).
Kashash, Y., Smarsh, G., Zilkha, N., Yovel, Y. & Kimchi, T. Alone, in the dark: the extraordinary neuroethology of the solitary blind mole rat. eLife 11, e78295 (2022).
Acknowledgements
This work was supported by the Wellcome Trust (212247/Z/18/Z) to M.P., the NIH (R01MH126676) to S.B. and the Lundbeckfonden grant (R361-2020-2654) to D.W.K.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Karine Rizzoti, Julie Chowen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Placzek, M., Chinnaiya, K., Kim, D.W. et al. Control of tuberal hypothalamic development and its implications in metabolic disorders. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-01036-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-024-01036-1