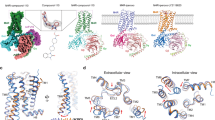
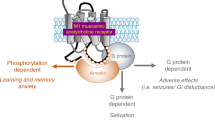
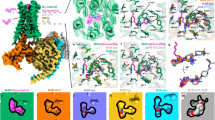
Abstract
Over the past 40 years, the muscarinic acetylcholine receptor family, particularly the M1-receptor and M4-receptor subtypes, have emerged as validated targets for the symptomatic treatment of neurological diseases such as schizophrenia and Alzheimer disease. However, despite considerable effort and investment, no drugs have yet gained clinical approval. This is largely attributable to cholinergic adverse effects that have halted the majority of programmes and resulted in a waning of interest in these G-protein-coupled receptor targets. Recently, this trend has been reversed. Driven by advances in structure-based drug design and an appreciation of the optimal pharmacological properties necessary to deliver clinical efficacy while minimizing adverse effects, a new generation of M1-receptor and M4-receptor orthosteric agonists and positive allosteric modulators are now entering the clinic. These agents offer the prospect of novel therapeutic solutions for ‘hard to treat’ neurological diseases, heralding a new era of muscarinic drug discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ellul-Micallef, R. History of Bronchial Asthma (Lippincott Raven, 1997).
Moulton, B. C. & Fryer, A. D. Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br. J. Pharmacol. 163, 44–52 (2011).
Singh, A., Dikshit, R. & Chaturvedi, P. Betel nut use: the South Asian story. Subst. Use Misuse 55, 1545–1551 (2020).
Sullivan, R. J., Allen, J. S., Otto, C., Tiobech, J. & Nero, K. Effects of chewing betel nut (Areca catechu) on the symptoms of people with schizophrenia in Palau, Micronesia. Br. J. Psychiatry 177, 174–178 (2000).
Feldberg, W. & Gaddum, J. H. The chemical transmitter at synapses in a sympathetic ganglion. J. Physiol. 81, 305–319 (1934).
Lopez-Munoz, F. & Alamo, C. Historical evolution of the neurotransmission concept. J. Neural Transm. 116, 515–533 (2009).
Felder, C. C. et al. Current status of muscarinic M1 and M4 receptors as drug targets for neurodegenerative diseases. Neuropharmacology 136, 449–458 (2018).
Bender, A. M., Jones, C. K. & Lindsley, C. W. Classics in chemical neuroscience: xanomeline. ACS Chem. Neurosci. 8, 435–443 (2017).
Budzik, B. et al. Novel N-substituted benzimidazolones as potent, selective, CNS-penetrant, and orally active M1 mAChR agonists. ACS Med. Chem. Lett. 1, 244–248 (2010).
Viberg, A., Martino, G., Lessard, E. & Laird, J. M. Evaluation of an innovative population pharmacokinetic-based design for behavioral pharmacodynamic endpoints. AAPS J. 14, 657–663 (2012).
Okada, H. et al. Alterations in α4β2 nicotinic receptors in cognitive decline in Alzheimer’s aetiopathology. Brain 136, 3004–3017 (2013).
Caulfield, M. P. Muscarinic receptors–characterization, coupling and function. Pharmacol. Ther. 58, 319–379 (1993).
Burford, N. T., Tobin, A. B. & Nahorski, S. R. Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 274, 134–142 (1995).
Burford, N. T., Tobin, A. B. & Nahorski, S. R. Coupling of muscarinic m1, m2 and m3 acetylcholine receptors, expressed in Chinese hamster ovary cells, to pertussis toxin-sensitive/insensitive guanine nucleotide-binding proteins. Eur. J. Pharmacol. 289, 343–351 (1995).
Gurevich, V. V. & Gurevich, E. V. GPCR signaling regulation: the role of GRKs and arrestins. Front. Pharmacol. 10, 125 (2019).
Bradley, S. J. et al. Biased M1-muscarinic-receptor-mutant mice inform the design of next-generation drugs. Nat. Chem. Biol. 16, 240–249 (2020).
Bradley, S. J. et al. Mapping physiological G protein-coupled receptor signaling pathways reveals a role for receptor phosphorylation in airway contraction. Proc. Natl Acad. Sci. USA 113, 4524–4529 (2016).
Butcher, A. J. et al. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 286, 11506–11518 (2011).
Budd, D. C., Willars, G. B., McDonald, J. E. & Tobin, A. B. Phosphorylation of the Gq/11-coupled m3-muscarinic receptor is involved in receptor activation of the ERK-1/2 mitogen-activated protein kinase pathway. J. Biol. Chem. 276, 4581–4587 (2001).
Lin, A. L. et al. Distinct pathways of ERK activation by the muscarinic agonists pilocarpine and carbachol in a human salivary cell line. Am. J. Physiol. Cell Physiol. 294, C1454–C1464 (2008).
Poulin, B. et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl Acad. Sci. USA 107, 9440–9445 (2010).
Kong, K. C. et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc. Natl Acad. Sci. USA 107, 21181–21186 (2010).
Smith, J. S., Lefkowitz, R. J. & Rajagopal, S. Biased signalling: from simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 17, 243–260 (2018).
Reiter, E., Ahn, S., Shukla, A. K. & Lefkowitz, R. J. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 52, 179–197 (2012).
Berizzi, A. E. et al. Muscarinic M5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology 43, 1510–1517 (2018).
Levey, A. I., Edmunds, S. M., Koliatsos, V., Wiley, R. G. & Heilman, C. J. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J. Neurosci. 15, 4077–4092 (1995).
Wess, J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 44, 423–450 (2004).
Mesulam, M., Shaw, P., Mash, D. & Weintraub, S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann. Neurol. 55, 815–828 (2004).
Davies, P. & Maloney, A. J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2, 1403 (1976).
Mufson, E. J., Counts, S. E., Perez, S. E. & Ginsberg, S. D. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev. Neurother. 8, 1703–1718 (2008).
Bartus, R. T., Dean, R. L. 3rd, Beer, B. & Lippa, A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–414 (1982).
Hampel, H. et al. Revisiting the cholinergic hypothesis in Alzheimer’s disease: emerging evidence from translational and clinical research. J. Prev. Alzheimers Dis. 6, 2–15 (2019).
Bartus, R. T. Physostigmine and recent memory: effects in young and aged nonhuman primates. Science 206, 1087–1089 (1979).
Bartus, R. T., Dean, R. L., Pontecorvo, M. J. & Flicker, C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann. N. Y. Acad. Sci. 444, 332–358 (1985).
Douchamps, V. & Mathis, C. A second wind for the cholinergic system in Alzheimer’s therapy. Behav. Pharmacol. 28, 112–123 (2017).
Courtney, C. et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 363, 2105–2115 (2004).
Inglis, F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 127, 45–63 (2002).
Thompson, S., Lanctot, K. L. & Herrmann, N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin. Drug Saf. 3, 425–440 (2004).
May, L. T., Leach, K., Sexton, P. M. & Christopoulos, A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51 (2007).
Chan, W. Y. et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl Acad. Sci. USA 105, 10978–10983 (2008).
Conn, P. J., Christopoulos, A. & Lindsley, C. W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 8, 41–54 (2009).
Bradley, S. J. et al. M1 muscarinic allosteric modulators slow prion neurodegeneration and restore memory loss. J. Clin. Invest. 127, 487–499 (2017).
Anagnostaras, S. G., Maren, S., Sage, J. R., Goodrich, S. & Fanselow, M. S. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology 21, 731–744 (1999).
Brown, A. J. H. et al. From structure to clinic: design of a muscarinic M1 receptor agonist with potential to treatment of Alzheimer’s disease. Cell 184, 5886–5901 e5822 (2021). Bench-to-bedside report using structure-based drug design to rationally generate a M1/M4-receptor agonist activating memory centres in elderly volunteers.
Digby, G. J. et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J. Neurosci. 32, 8532–8544 (2012). Study showing that M1 receptors in different brain regions are differentially affected by M1-receptor PAMs.
Ghoshal, A. et al. Potentiation of M1 muscarinic receptor reverses plasticity deficits and negative and cognitive symptoms in a schizophrenia mouse model. Neuropsychopharmacology 41, 598–610 (2016).
Moran, S. P. et al. M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology 43, 1763–1771 (2018).
Rook, J. M. et al. A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem. Neurosci. 9, 2274–2285 (2018).
Shirey, J. K. et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 29, 14271–14286 (2009).
Conley, A. C. et al. Cognitive performance effects following a single dose of the M1 muscarinic positive allosteric modulator VU319. Alzheimers Dement.16, e045339 (2020).
Ma, L. et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl Acad. Sci. USA 106, 15950–15955 (2009). Early demonstration of the cognitive properties of M1-receptor PAMs.
Anagnostaras, S. G. et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 6, 51–58 (2003).
Lebois, E. P. et al. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M(1) receptor function in the central nervous system. ACS Chem. Neurosci. 1, 104–121 (2010).
Lebois, E. P. et al. Development of a highly selective, orally bioavailable and CNS penetrant M1 agonist derived from the MLPCN probe ML071. Bioorg. Med. Chem. Lett. 21, 6451–6455 (2011).
Scheiderer, C. L. et al. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3-CA1 synapses. J. Neurosci. 26, 3745–3756 (2006).
Shinoe, T., Matsui, M., Taketo, M. M. & Manabe, T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J. Neurosci. 25, 11194–11200 (2005).
Miyakawa, T., Yamada, M., Duttaroy, A. & Wess, J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 21, 5239–5250 (2001).
Gerber, D. J. et al. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl Acad. Sci. USA 98, 15312–15317 (2001).
Salah-Uddin, H. et al. Altered M(1) muscarinic acetylcholine receptor (CHRM1)-Galpha(q/11) coupling in a schizophrenia endophenotype. Neuropsychopharmacology 34, 2156–2166 (2009).
Foster, D. J., Choi, D. L., Conn, P. J. & Rook, J. M. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 10, 183–191 (2014).
Foster, D. J. et al. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron 91, 1244–1252 (2016).
Gogliotti, R. G. et al. Total RNA sequencing of Rett syndrome autopsy samples identifies the M4 muscarinic receptor as a novel therapeutic target. J. Pharmacol. Exp. Ther. 365, 291–300 (2018).
Tarr, J. C. et al. Challenges in the development of an M4 PAM preclinical candidate: the discovery, SAR, and in vivo characterization of a series of 3-aminoazetidine-derived amides. Bioorg. Med. Chem. Lett. 27, 2990–2995 (2017).
Woolley, M. L., Carter, H. J., Gartlon, J. E., Watson, J. M. & Dawson, L. A. Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur. J. Pharmacol. 603, 147–149 (2009).
Yohn, S. E. & Conn, P. J. Positive allosteric modulation of M1 and M4 muscarinic receptors as potential therapeutic treatments for schizophrenia. Neuropharmacology 136, 438–448 (2017).
Felder, C. C. et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 68, 2605–2613 (2001).
Thomsen, M., Wess, J., Fulton, B. S., Fink-Jensen, A. & Caine, S. B. Modulation of prepulse inhibition through both M1 and M4 muscarinic receptors in mice. Psychopharmacology 208, 401–416 (2010).
Tzavara, E. T. et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 18, 1410–1412 (2004).
Foster, D. J., Bryant, Z. K. & Conn, P. J. Targeting muscarinic receptors to treat schizophrenia. Behav. Brain Res. 405, 113201 (2021).
Paul, S. M., Yohn, S. E., Popiolek, M., Miller, A. C. & Felder, C. C. Muscarinic acetylcholine receptor agonists as novel treatments for schizophrenia. Am. J. Psychiatry 179, 611–627 (2022).
Lange, H. S. et al. Effects of a novel M4 muscarinic positive allosteric modulator on behavior and cognitive deficits relevant to Alzheimer’s disease and schizophrenia in rhesus monkey. Neuropharmacology 197, 108754 (2021).
Bubser, M. et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci. 5, 920–942 (2014).
Gould, R. W. et al. Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154. Neuropharmacology 128, 492–502 (2018).
Jeon, J. et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J. Neurosci. 30, 2396–2405 (2010).
Bymaster, F. P., Felder, C., Ahmed, S. & McKinzie, D. Muscarinic receptors as a target for drugs treating schizophrenia. Curr. Drug Targets CNS Neurol. Disord. 1, 163–181 (2002).
Conn, P. J., Jones, C. K. & Lindsley, C. W. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 30, 148–155 (2009).
Heinrich, J. N. et al. Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists. Eur. J. Pharmacol. 605, 53–56 (2009).
Shannon, H. E. et al. Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors. J. Pharmacol. Exp. Ther. 269, 271–281 (1994).
Bodick, N. C. et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 54, 465–473 (1997).
Bodick, N. C. et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 11 (Suppl. 4), S16–S22 (1997).
Shekhar, A. et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 1033–1039 (2008).
Andersen, M. B. et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in Cebus apella monkeys. Neuropsychopharmacology 28, 1168–1175 (2003).
Sramek, J. J. et al. The safety and tolerance of xanomeline tartrate in patients with Alzheimer’s disease. J. Clin. Pharmacol. 35, 800–806 (1995).
Burger, W. A. C. et al. Xanomeline displays concomitant orthosteric and allosteric binding modes at the M(4) mAChR. Nat. Commun. 14, 5440 (2023).
Thal, D. M. et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531, 335–340 (2016).
Vuckovic, Z. et al. Crystal structure of the M5 muscarinic acetylcholine receptor. Proc. Natl Acad. Sci. USA 116, 26001–26007 (2019).
Kaul, I. et al. Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA Psychiatry https://doi.org/10.1001/jamapsychiatry.2024.0785 (2024).
Kaul, I. et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 403, 160–170 (2024). Phase III clinical trial of KarXT, a combination therapy containing the partial muscarinic agonist xanomeline with the peripherally restricted antagonist trospium marking a breakthrough medicine in the treatment of SZ.
Bradley, S. J. et al. Bitopic binding mode of an M1 muscarinic acetylcholine receptor agonist associated with adverse clinical trial outcomes. Mol. Pharm. 93, 645–656 (2018).
Keov, P., Sexton, P. M. & Christopoulos, A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60, 24–35 (2011).
Langmead, C. J. & Christopoulos, A. Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends Pharmacol. Sci. 27, 475–481 (2006).
Canals, M. et al. A Monod-Wyman-Changeux mechanism can explain G protein-coupled receptor (GPCR) allosteric modulation. J. Biol. Chem. 287, 650–659 (2012).
Wootten, D., Christopoulos, A. & Sexton, P. M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat. Rev. Drug Discov. 12, 630–644 (2013).
Rasmussen, S. G. et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450, 383–387 (2007). First atomic-level strucutre of a non-visual GPCR launching the prospect of structure-based drug design for GPCR ligands.
Rosenbaum, D. M. et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318, 1266–1273 (2007).
Cherezov, V. et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Noble, M. E., Endicott, J. A. & Johnson, L. N. Protein kinase inhibitors: insights into drug design from structure. Science 303, 1800–1805 (2004).
Kruse, A. C. et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–556 (2012).
Haga, K. et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 482, 547–551 (2012).
Warne, T. et al. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 (2008).
Bakker, C. et al. Safety and pharmacokinetics of HTL0018318, a novel M1 receptor agonist, given in combination with donepezil at steady state: a randomized trial in healthy elderly subjects. Drugs R D 21, 295–304 (2021).
Nathan, P. J. et al. A phase 1b/2a multicenter study of the safety and preliminary pharmacodynamic effects of selective muscarinic M1 receptor agonist HTL0018318 in patients with mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 8, e12273 (2022).
Vuckovic, Z. et al. Pharmacological hallmarks of allostery at the M4 muscarinic receptor elucidated through structure and dynamics. eLife 12, e83477 (2023).
Thal, D. M., Glukhova, A., Sexton, P. M. & Christopoulos, A. Structural insights into G-protein-coupled receptor allostery. Nature 559, 45–53 (2018).
Burger, W. A. C., Sexton, P. M., Christopoulos, A. & Thal, D. M. Toward an understanding of the structural basis of allostery in muscarinic acetylcholine receptors. J. Gen. Physiol. 150, 1360–1372 (2018).
Nawaratne, V., Leach, K., Felder, C. C., Sexton, P. M. & Christopoulos, A. Structural determinants of allosteric agonism and modulation at the M4 muscarinic acetylcholine receptor: identification of ligand-specific and global activation mechanisms. J. Biol. Chem. 285, 19012–19021 (2010).
Kruse, A. C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013). Breakthrough study describing the co-crystal structure of a mAChR complexed with an orthosteric and allosteric modulator.
Valant, C., Felder, C. C., Sexton, P. M. & Christopoulos, A. Probe dependence in the allosteric modulation of a G protein-coupled receptor: implications for detection and validation of allosteric ligand effects. Mol. Pharmacol. 81, 41–52 (2012).
Foster, D. J. & Conn, P. J. Allosteric modulation of GPCRs: new insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron 94, 431–446 (2017).
Nickols, H. H. & Conn, P. J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol. Dis. 61, 55–71 (2014).
Beshore, D. C. et al. MK-7622: a first-in-class M1 positive allosteric modulator development candidate. ACS Med. Chem. Lett. 9, 652–656 (2018).
Davoren, J. E. et al. Design and synthesis of gamma- and delta-lactam M1 positive allosteric modulators (PAMs): convulsion and cholinergic toxicity of an M1-selective PAM with weak agonist activity. J. Med. Chem. 60, 6649–6663 (2017).
Davoren, J. E. et al. Discovery of the potent and selective M1 PAM-agonist N-[(3 R,4 S)-3-hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)ben zyl]pyridine-2-carboxamide (PF-06767832): evaluation of efficacy and cholinergic side effects. J. Med Chem. 59, 6313–6328 (2016).
Davoren, J. E. et al. Design and optimization of selective azaindole amide M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 26, 650–655 (2016).
Voss, T. et al. Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimers Dement. 4, 173–181 (2018).
Rook, J. M. et al. Diverse effects on M1 signaling and adverse effect liability within a series of M1 Ago-PAMs. ACS Chem. Neurosci. 8, 866–883 (2017). Demonstration of differential adverse effects of M1-receptor PAMs with various levels of intrinsic activity.
Newhouse, P. A. et al. Development of the muscarinic cholinergic PAM VU319 for cognitive enhencement: phase 1 tests of safety and target engagement. Alzheimers Dement. 15, P574 (2019).
Digby, G. J., Shirey, J. K. & Conn, P. J. Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol. BioSyst. 6, 1345–1354 (2010).
Melancon, B. J. et al. Optimization of M4 positive allosteric modulators (PAMs): the discovery of VU0476406, a non-human primate in vivo tool compound for translational pharmacology. Bioorg. Med. Chem. Lett. 27, 2296–2301 (2017).
Wood, M. R. et al. Discovery of VU0467485/AZ13713945: an M4 PAM evaluated as a preclinical candidate for the treatment of schizophrenia. ACS Med. Chem. Lett. 8, 233–238 (2017).
Marlo, J. E. et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. 75, 577–588 (2009).
Shirey, J. K. et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 4, 42–50 (2008).
Brady, A. E. et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J. Pharmacol. Exp. Ther. 327, 941–953 (2008).
Wood, M. R. et al. Challenges in the development of an M4 PAM in vivo tool compound: the discovery of VU0467154 and unexpected DMPK profiles of close analogs. Bioorg. Med. Chem. Lett. 27, 171–175 (2017).
Engers, D. W. et al. VU6005806/AZN-00016130, an advanced M4 positive allosteric modulator (PAM) profiled as a potential preclinical development candidate. Bioorg. Med. Chem. Lett. 29, 1714–1718 (2019).
Krystal, J. H. et al. Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part, randomised, double-blind, placebo-controlled, phase 1b trial. Lancet 400, 2210–2220 (2022).
Urban, D. J. & Roth, B. L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007). First description of the widely used muscarinic receptor chemogenetic variant called a DREADD.
Alt, A. et al. Evidence for classical cholinergic toxicity associated with selective activation of m1 muscarinic receptors. J. Pharmacol. Exp. Ther. 356, 293–304 (2016).
Kenakin, T. & Christopoulos, A. Analytical pharmacology: the impact of numbers on pharmacology. Trends Pharmacol. Sci. 32, 189–196 (2011).
Kenakin, T., Watson, C., Muniz-Medina, V., Christopoulos, A. & Novick, S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 3, 193–203 (2012).
Porter, A. C. et al. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 944, 82–89 (2002).
Lebois, E. P., Thorn, C., Edgerton, J. R., Popiolek, M. & Xi, S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 136, 362–373 (2018).
Potter, P. E. et al. Pre- and post-synaptic cortical cholinergic deficits are proportional to amyloid plaque presence and density at preclinical stages of Alzheimer’s disease. Acta Neuropathol. 122, 49–60 (2011).
Kenakin, T. & Christopoulos, A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 12, 205–216 (2013).
Donthamsetti, P. et al. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol. Psychiatry 25, 2086–2100 (2020).
Bradley, S. J. & Tobin, A. B. Design of next-generation G protein-coupled receptor drugs: linking novel pharmacology and in vivo animal models. Annu. Rev. Pharmacol. Toxicol. 56, 535–559 (2016).
Barch, D. M. et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch. Gen. Psychiatry 58, 280–288 (2001).
Allen, G. et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch. Neurol. 64, 1482–1487 (2007).
Nitsch, R. M., Slack, B. E., Wurtman, R. J. & Growdon, J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258, 304–307 (1992).
Farber, S. A., Nitsch, R. M., Schulz, J. G. & Wurtman, R. J. Regulated secretion of beta-amyloid precursor protein in rat brain. J. Neurosci. 15, 7442–7451 (1995).
Hock, C. et al. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of Aβ 42 in patients with Alzheimer’s disease. Amyloid 10, 1–6 (2003).
Nitsch, R. M., Deng, M., Tennis, M., Schoenfeld, D. & Growdon, J. H. The selective muscarinic M1 agonist AF102B decreases levels of total Aβ in cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 48, 913–918 (2000).
Davis, A. A., Fritz, J. J., Wess, J., Lah, J. J. & Levey, A. I. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J. Neurosci. 30, 4190–4196 (2010).
Medeiros, R. et al. Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am. J. Pathol. 179, 980–991 (2011).
Jones, C. K. et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 28, 10422–10433 (2008). Early demonstration that M1 receptors can regulate the processing of APP and thereby potentially slow the progression of neurodegenerative disease.
Caccamo, A. et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 49, 671–682 (2006).
Lebois, E. P. et al. Disease-modifying effects of M1 muscarinic acetylcholine receptor activation in an Alzheimer’s disease mouse model. ACS Chem. Neurosci. 8, 1177–1187 (2017).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706 (1991).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375, 754–760 (1995).
Karran, E. & De Strooper, B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21, 306–318 (2022).
Karran, E., Mercken, M. & De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 (2011).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–1938 (2015).
Yiannopoulou, K. G., Anastasiou, A. I., Zachariou, V. & Pelidou, S. H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 7, 97 (2019).
Lowe, V. J. et al. Cross-sectional associations of tau-PET signal with cognition in cognitively unimpaired adults. Neurology 93, e29–e39 (2019).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014).
Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 19, 687–700 (2018).
Pontecorvo, M. J. et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140, 748–763 (2017).
Halliday, M., Radford, H. & Mallucci, G. R. Prions: generation and spread versus neurotoxicity. J. Biol. Chem. 289, 19862–19868 (2014).
Mallucci, G. Spreading proteins in neurodegeneration: where do they take us? Brain 136, 994–995 (2013).
Dwomoh, L. et al. M1 muscarinic receptor activation reduces the molecular pathology and slows the progression of prion-mediated neurodegenerative disease. Sci. Signal. 15, eabm3720 (2022).
Scarpa, M. et al. Biased M1 muscarinic receptor mutant mice show accelerated progression of prion neurodegenerative disease. Proc. Natl Acad. Sci. USA 118, e2107389118 (2021).
Gunter, B. W. et al. Selective inhibition of M5 muscarinic acetylcholine receptors attenuates cocaine self-administration in rats. Addict. Biol. 23, 1106–1116 (2018).
Basile, A. S. et al. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc. Natl Acad. Sci. USA 99, 11452–11457 (2002). Study describing the potential for M5-receptor inhibitors to prevent the acquisition of addiction to substances of abuse, including opioids.
Steidl, S., Miller, A. D., Blaha, C. D. & Yeomans, J. S. M5 muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PloS ONE 6, e27538 (2011).
Teal, L. B. et al. Selective M(5) muscarinic acetylcholine receptor negative allosteric modulator VU6008667 blocks acquisition of opioid self-administration. Neuropharmacology 227, 109424 (2023).
Gould, R. W. et al. Acute negative allosteric modulation of M5 muscarinic acetylcholine receptors inhibits oxycodone self-administration and cue-induced reactivity with no effect on antinociception. ACS Chem. Neurosci. 10, 3740–3750 (2019).
McGowan, K. M. et al. Continued optimization of the M5 NAM ML375: discovery of VU6008667, an M5 NAM with high CNS penetration and a desired short half-life in rat for addiction studies. Bioorg. Med. Chem. Lett. 27, 1356–1359 (2017).
Garrison, A. T. et al. Development of VU6019650: a potent, highly selective, and systemically active orthosteric antagonist of the M5 muscarinic acetylcholine receptor for the treatment of opioid use disorder. J. Med. Chem. 65, 6273–6286 (2022).
Nunes, E. J. et al. Examining the role of muscarinic M5 receptors in VTA cholinergic modulation of depressive-like and anxiety-related behaviors in rats. Neuropharmacology 171, 108089 (2020).
Nunes, E. J. et al. Ventral tegmental area M5 muscarinic receptors mediate effort-choice responding and nucleus accumbens dopamine in a sex-specific manner. J. Pharmacol. Exp. Ther. 385, 146–156 (2023).
Dulawa, S. C. & Janowsky, D. S. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol. Psychiatry 24, 694–709 (2019).
Janowsky, D. S., el-Yousef, M. K. & Davis, J. M. Acetylcholine and depression. Psychosom. Med. 36, 248–257 (1974).
Leach, K. et al. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology 35, 855–869 (2010).
Black, J. W. & Leff, P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B 220, 141–162 (1983).
Brannan, S. K. et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N. Engl. J. Med. 384, 717–726 (2021).
Acknowledgements
The author thanks the Wellcome Trust, who provided a Collaborative Award (201529/Z/16/Z) to A.B.T., and the generous donations of the Rice family, and Alan and Ann Boyd. Thanks also to C. Jones (Vanderbilt University) for proofreading and advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A.T. is fully employed by the University of Glasgow but is also co-founder and CEO of the spin-out company Keltic Pharma Therapeutics Ltd, which has an interest in targeting muscarinic receptors in neurological disease.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Jan Jakubik, Daniel Foster and Christian Felder for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tobin, A.B. A golden age of muscarinic acetylcholine receptor modulation in neurological diseases. Nat Rev Drug Discov (2024). https://doi.org/10.1038/s41573-024-01007-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-024-01007-1