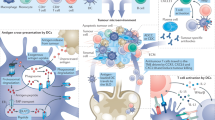
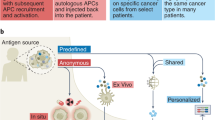
Abstract
mRNA vaccines have been revolutionary in terms of their rapid development and prevention of SARS-CoV-2 infections during the COVID-19 pandemic, and this technology has considerable potential for application to the treatment of cancer. Compared with traditional cancer vaccines based on proteins or peptides, mRNA vaccines reconcile the needs for both personalization and commercialization in a manner that is unique to each patient but not beholden to their HLA haplotype. A further advantage of mRNA vaccines is the availability of engineering strategies to improve their stability while retaining immunogenicity, enabling the induction of complementary innate and adaptive immune responses. Thus far, no mRNA-based cancer vaccines have received regulatory approval, although several phase I–II trials have yielded promising results, including in historically poorly immunogenic tumours. Furthermore, many early phase trials testing a wide range of vaccine designs are currently ongoing. In this Review, we describe the advantages of cancer mRNA vaccines and advances in clinical trials using both cell-based and nanoparticle-based delivery methods, with discussions of future combinations and iterations that might optimize the activity of these agents.
Key points
-
The advent of mRNA-based vaccines against SARS-CoV-2 has ushered in a new era of mRNA vaccines against other infectious diseases as well as cancer.
-
Advantages of directly injected mRNA vaccines include safety and flexibility in terms of the speed with which personalized epitopes or antigens can be produced in the form of mRNA.
-
mRNA is a labile molecule and is best delivered in nanoparticles, which are not currently developed to target specific cell types for the induction of immune responses. Alternatively, mRNA can be loaded into antigen-presenting cells that can then be administered as a vaccine.
-
SARS-CoV-2 vaccines use mRNA incorporating modified nucleotides to minimize the risk of a type I interferon response. However, this response might be beneficial in the setting of cancer vaccines, which must generate immune responses against antigens that are overexpressed self-antigens or those that result in only slightly altered proteins and are therefore likely to be less immunogenic.
-
The future of mRNA vaccines will include optimization of the nanoparticles used to deliver the vaccine as well as of the mRNA itself.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26, 154–158 (2006).
van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16, 219–233 (2016).
Pollack, I. F. et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 32, 2050–2058 (2014).
Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 4722–4729 (2010).
Prins, R. M., Cloughesy, T. F. & Liau, L. M. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N. Engl. J. Med. 359, 539–541 (2008).
Shi, G. N. et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 113, 191–202 (2017).
Duraiswamy, J., Kaluza, K. M., Freeman, G. J. & Coukos, G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 73, 3591–3603 (2013).
Coban, C. et al. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther. 11, 479–484 (2011).
Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force Members.Operation warp speed: implications for global vaccine security. Lancet Glob. Health 9, e1017–e1021 (2021).
U.S. Department of Health and Human Services. Explaining Operation Warp Speed https://www.nihb.org/covid-19/wp-content/uploads/2020/08/Fact-sheet-operation-warp-speed.pdf (accessed May 2024).
Mikulic, M. Number of COVID-19 vaccine doses administered in the United States as of April 26, 2023, by vaccine manufacturer. statista, https://www.statista.com/statistics/1198516/covid-19-vaccinations-administered-us-by-company/ (accessed May 2024).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
Yang, A., Farmer, E., Wu, T. C. & Hung, C. F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 23, 75 (2016).
Lei, J. et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 383, 1340–1348 (2020).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Navid, F. et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 32, 1445–1452 (2014).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Riedmann, E. M. World’s first cancer vaccine licensed: PROVENGE®. Hum. Vaccin. 6, 430–435 (2010).
Bot, A. The landmark approval of Provenge, what it means to immunology and “in this issue”: the complex relation between vaccines and autoimmunity. Int. Rev. Immunol. 29, 235–238 (2010).
Brower, V. Approval of provenge seen as first step for cancer treatment vaccines. J. Natl Cancer Inst. 102, 1108–1110 (2010).
Jahnisch, H. et al. Dendritic cell-based immunotherapy for prostate cancer. Clin. Dev. Immunol. 2010, 517493 (2010).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Paller, C. J. & Antonarakis, E. S. Sipuleucel-T for the treatment of metastatic prostate cancer: promise and challenges. Hum. Vaccin. Immunother. 8, 509–519 (2012).
Sheikh, N. A. et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 62, 137–147 (2013).
Sanchez-Perez, L. et al. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 65, 2009–2017 (2005).
Bloch, O. et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 19, 3165–3175 (2013).
Morse, M. A. et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 59, 56–58 (1999).
Quillien, V. et al. Biodistribution of radiolabelled human dendritic cells injected by various routes. Eur. J. Nucl. Med. Mol. Imaging 32, 731–741 (2005).
Bandola-Simon, J. & Roche, P. A. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol. 113, 31–37 (2019).
Demoulin, S., Herfs, M., Delvenne, P. & Hubert, P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J. Leukoc. Biol. 93, 343–352 (2013).
Oble, D. A., Loewe, R., Yu, P. & Mihm, M. C. Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 9, 3 (2009).
Hutchison, S. et al. Characterization of myeloid-derived suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with malignant melanoma receiving a GD3-based immunotherapy. Vet. Immunol. Immunopathol. 216, 109912 (2019).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Lohr, J. et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 17, 4296–4308 (2011).
Hwang, S. L., Chung, N. P., Chan, J. K. & Lin, C. L. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 15, 167–175 (2005).
Toda, Y. et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J. Cancer Res. Clin. Oncol. 146, 2607–2620 (2020).
Wainwright, D. A. et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 18, 6110–6121 (2012).
Tavernier, G. et al. mRNA as gene therapeutic: how to control protein expression. J. Control. Rel. 150, 238–247 (2011).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Martinon, F. et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 23, 1719–1722 (1993).
Conry, R. M. et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55, 1397–1400 (1995).
Johanning, F. W. et al. A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res. 23, 1495–1501 (1995).
Aliahmad, P., Miyake-Stoner, S. J., Geall, A. J. & Wang, N. S. Next generation self-replicating RNA vectors for vaccines and immunotherapies. Cancer Gene Ther. 30, 785–793 (2023).
Morse, M. A. et al. Clinical trials of self-replicating RNA-based cancer vaccines. Cancer Gene Ther. 30, 803–811 (2023).
Ulmer, J. B., Mason, P. W., Geall, A. & Mandl, C. W. RNA-based vaccines. Vaccine 30, 4414–4418 (2012).
Schlake, T., Thess, A., Fotin-Mleczek, M. & Kallen, K. J. Developing mRNA-vaccine technologies. RNA Biol. 9, 1319–1330 (2012).
Boczkowski, D., Nair, S. K., Nam, J. H., Lyerly, H. K. & Gilboa, E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 60, 1028–1034 (2000).
Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184, 465–472 (1996).
Nair, S. K. et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat. Biotechnol. 16, 364–369 (1998).
Heiser, A. et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest. 109, 409–417 (2002).
Van Tendeloo, V. F. et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98, 49–56 (2001).
Zhang, S. N. et al. Optimizing DC vaccination by combination with oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol. Ther. 19, 1558–1568 (2011).
Khoury, H. J. et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer 123, 3061–3072 (2017).
Anguille, S. et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 130, 1713–1721 (2017).
Boudewijns, S. et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunol. Immun. 69, 477–488 (2020).
Kongsted, P. et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: a randomized phase II study. Cytotherapy 19, 500–513 (2017).
Batich, K. A., Mitchell, D. A., Healy, P., Herndon, J. E. & Sampson, J. H. Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin. Cancer Res. 26, 5297–5303 (2020).
Mitchell, D. A. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369 (2015).
Batich, K. A. et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin. Cancer Res. 23, 1898–1909 (2017).
Batich, K. A., Swartz, A. M. & Sampson, J. H. Preconditioning vaccine sites for mRNA-transfected dendritic cell therapy and antitumor efficacy. Methods Mol. Biol. 1403, 819–838 (2016).
Wilgenhof, S. et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 34, 1330 (2016).
Dannull, J. et al. Melanoma immunotherapy using mature DCs expressing the constitutive proteasome. J. Clin. Invest. 123, 3135–3145 (2013).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
Su, Z. et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 63, 2127–2133 (2003).
Heiser, A. et al. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res. 61, 3388–3393 (2001).
Caruso, D. A. et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 6, 236–246 (2004).
Sayour, E. J. et al. Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett. 18, 6195–6206 (2018).
Nair, S. K. et al. Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J. Neurooncol. 125, 65–74 (2015).
Figlin, R. A. et al. Results of the ADAPT phase 3 study of rocapuldencel-T in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 26, 2327–2336 (2020).
DeBenedette, M., Gamble, A., Norris, M., Horvatinovich, J. & Nicolette, C. A. A review of the clinical experience with CMN-001, a tumor RNA loaded dendritic cell immunotherapy for the treatment of metastatic renal cell carcinoma. Hum. Vaccin. Immunother. 19, 2220629 (2023).
Lorentzen, C. L., Haanen, J. B., Met, O. & Svane, I. M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 23, e450–e458 (2022).
Benteyn, D., Heirman, C., Bonehill, A., Thielemans, K. & Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccin. 14, 161–176 (2015).
Nair, S., Boczkowski, D., Pruitt, S. & Urban, J. in Cancer Vaccines: From Research to Clinical Practice 1st ed (Eds Bot, A., Obrocea, M. & Marincola, F. M.) 217–231 (2011).
Dorrie, J., Schaft, N., Schuler, G. & Schuler-Thurner, B. Therapeutic cancer vaccination with ex vivo RNA-transfected dendritic cells-an update. Pharmaceutics 12, 92 (2020).
Van Driessche, A. et al. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy 11, 653–668 (2009).
Grabbe, S. et al. Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine 11, 2723–2734 (2016).
Oberli, M. A. et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 17, 1326–1335 (2017).
Sayour, E. J. et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. OncoImmunology 6, e1256527 (2016).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Mendez-Gomez, H. R. et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell 187, 2521–2535.e21 (2024).
Weide, B. et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 32, 498–507 (2009).
Papachristofilou, A. et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 7, 38 (2019).
Kubler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer 3, 26 (2015).
Cafri, G. et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 130, 5976–5988 (2020).
Weber, J. S. et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet 403, 632–644 (2024).
Raimondo, T. M., Reed, K., Shi, D. N., Langer, R. & Anderson, D. G. Delivering the next generation of cancer immunotherapies with RNA. Cell 186, 1535–1540 (2023).
Palmer, C. D. et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med. 28, 1619–1629 (2022).
Rappaport, A. R. et al. A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med. 30, 1013–1022 (2024).
Cruz, L. J. et al. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 509, 143–163 (2012).
Briquez, P. S. et al. Engineering targeting materials for therapeutic cancer vaccines. Front. Bioeng. Biotechnol. 8, 19 (2020).
Eigentler, T. et al. A phase I dose-escalation and expansion study of intratumoral CV8102 as single-agent or in combination with anti-PD-1 antibodies in patients with advanced solid tumors. J. Clin. Oncol. 38, https://doi.org/10.1200/JCO.2020.38.15_suppl.3096 (2020).
Eigentler, T. et al. Intratumorally administered CV8102 in patients with advanced solid tumors: preliminary results from ongoing expansion part in study 008. J. Immunother. Cancer 10, A818 (2022).
Patel, M. R. et al. A phase I study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral (iTu) injection alone and in combination with durvalumab. J. Clin. Oncol. 38, 3092 (2020).
Jimeno, A. et al. A phase 1/2, open-label, multicenter, dose escalation and efficacy study of mRNA-2416, a lipid nanoparticle encapsulated mRNA encoding human OX40L, for intratumoral injection alone or in combination with durvalumab for patients with advanced malignancies. Cancer Res. 80, CT032 (2020).
Bechter, O. et al. Abstract LB198: a first-in-human, open-label, multicenter study of intratumoral SAR441000 (mixture of cytokine encoding mRNAs), as monotherapy and in combination with cemiplimab in patients with advanced solid tumors. Phase 1/2 study of mRNA-4359 administered alone and in combination with immune checkpoint blockade in adult participants with advanced solid tumors. Cancer Res. https://doi.org/10.1158/1538-7445.AM2023-LB198 (2023).
Barral, P. M. et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol. Ther. 124, 219–234 (2009).
Kang, D. C. et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23, 1789–1800 (2004).
Yoneyama, M. et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 (2005).
Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008).
Zhang, Y. L., Guo, Y. J., Bin, L. & Sun, S. H. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J. Hepatol. 51, 29–38 (2009).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M.Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Chattopadhyay, S. & Sen, G. C. dsRNA-activation of TLR3 and RLR signaling: gene induction-dependent and independent effects. J. Interferon Cytokine Res. 34, 427–436 (2014).
Broos, K. et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol. Ther. Nucleic Acids 5, e326 (2016).
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 (2005).
Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383, 2427–2438 (2020).
Sittplangkoon, C. et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 13, 983000 (2022).
Ramos da Silva, J. et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 15, eabn3464 (2023).
Holtkamp, S. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108, 4009–4017 (2006).
Bonehill, A. et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J. Immunol. 172, 6649–6657 (2004).
Su, Y., Connolly, M., Marketon, A. & Heiland, T. CryJ-LAMP DNA vaccines for Japanese red edar allergy induce robust Th1-type immune responses in murine model. J. Immunol. Res. 2016, 4857869 (2016).
Beck, J. D. et al. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 20, 69 (2021).
Orlandini von Niessen, A. G. et al. Improving mRNA-Based therapeutic gene delivery by expression-augmenting 3’ UTRs identified by cellular library screening. Mol. Ther. 27, 824–836 (2019).
Niu, D., Wu, Y. & Lian, J. Circular RNA vaccine in disease prevention and treatment. Signal. Transduct. Target. Ther. 8, 341 (2023).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med. 29, 2844–2853 (2023).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Yap, T. A. et al. A phase I/II dose escalation trial with expansion cohorts to evaluate safety and preliminary efficacy of BNT142 in patients with prospectively confirmed claudin 6-positive solid tumors J. Clin. Oncol. 41, https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS2669 (2023).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Lopez, J. S. et al. A phase Ib study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in combination with atezolizumab in patients with locally advanced or metastatic solid tumors. Cancer Res. 80, CT301 (2020).
Castañón, E. et al. Intratumoral (IT) MEDI1191 + durvalumab (D): update on the first-in-human study in advanced solid tumors. Cancer Res. 83, CT004 (2023).
Author information
Authors and Affiliations
Contributions
E.J.S., D.B. and S.K.N. researched data for the manuscript. E.J.S. and D.B. wrote the manuscript. All authors made a substantial contribution to discussions of content and reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
E.J.S. has acted as a consultant of Siren Biotechnology and is listed on pending patent applications relating to technologies discussed in this manuscript that are optioned to license to iOncologi, Inc. D.A.M. is listed on pending patent applications on technologies discussed in this manuscript that are optioned to license to iOncologi, Inc. and holds ownership interests in iOncologi, Inc. S.K.N. and D.B. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks Z. Berneman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayour, E.J., Boczkowski, D., Mitchell, D.A. et al. Cancer mRNA vaccines: clinical advances and future opportunities. Nat Rev Clin Oncol (2024). https://doi.org/10.1038/s41571-024-00902-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41571-024-00902-1