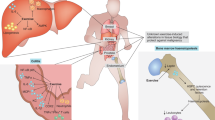
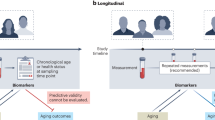
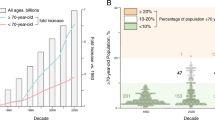
Abstract
A growing body of evidence indicates that patients with cancer who receive cytotoxic treatments (such as chemotherapy or radiotherapy) have an increased risk of accelerated physical and cognitive ageing. Furthermore, accelerated biological ageing is a suspected driving force behind many of these observed effects. In this Review, we describe the mechanisms of biological ageing and how they apply to patients with cancer. We highlight the important role of specific behavioural factors, namely stress, sleep and lifestyle-related factors such as physical activity, weight management, diet and substance use, in the accelerated ageing of patients with cancer and cancer survivors. We also present a framework of how modifiable behaviours could operate to either increase the risk of accelerated ageing, provide protection, or promote resilience at both the biological level and in terms of patient-reported outcomes.
Key points
-
Cancer and its treatments are thought to promote accelerated biological ageing, leading to adverse cognitive, behavioural and functional outcomes in cancer survivors.
-
Modifiable host-specific factors are known to affect ageing biology in individuals without cancer, including psychosocial stress, poor sleep, physical inactivity, obesity, and tobacco and alcohol use.
-
Behavioural interventions and/or modifications targeting these host factors might directly alter biological ageing processes in patients with cancer and cancer survivors, thereby improving both healthspan and lifespan.
-
We propose that these host factors be considered in models of cancer-related age acceleration and that interventions designed to reduce stress, improve sleep health, increase physical activity, manage weight, and/or reduce alcohol and tobacco use be investigated as promising approaches to address accelerated ageing in this context.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guida, J. L. et al. Measuring aging and identifying aging phenotypes in cancer survivors. J. Natl Cancer Inst. 111, 1245–1254 (2019).
Henderson, T. O., Ness, K. K. & Cohen, H. J. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am. Soc. Clin. Oncol. Educ. B. 34, e423–e430 (2014).
Maccormick, R. E. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med. Hypotheses 67, 212–215 (2006).
Bluethmann, S. M., Mariotto, A. B. & Rowland, J. H. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 25, 1029–1036 (2016).
Wood, W. A. et al. Chemotherapy and stem cell transplantation increase p16INK4a expression, a biomarker of T-cell aging. EBioMedicine 11, 227–238 (2016).
Ness, K. K. et al. Frailty in childhood cancer survivors. Cancer 121, 1540–1547 (2015).
Ness, K. K. et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude lifetime cohort study. J. Clin. Oncol. 31, 4496–4503 (2013).
Mandelblatt, J. S. et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin. Oncol. 40, 709–725 (2013).
Cupit-Link, M. C. et al. Biology of premature ageing in survivors of cancer. ESMO Open 2, e000250 (2017).
Goedendorp, M. M. et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer 118, 3833–3841 (2012).
Janelsins, M. C. et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol. 35, 506–514 (2017).
Bower, J. E. et al. Fatigue in long-term breast carcinoma survivors. Cancer 106, 751–758 (2006).
Ganz, P. A. et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J. Natl Cancer Inst. 94, 39–49 (2002).
Ganz, P. A., Rowland, J. H., Meyerowitz, B. E. & Desmond, K. A. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 152, 396–411 (1998).
Ahles, T. A., Root, J. C. & Ryan, E. L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol. 30, 3675–3686 (2012).
Sehl, M. E. & Ganz, P. A. Potential mechanisms of age acceleration caused by estrogen deprivation: do endocrine therapies carry the same risks? JNCI Cancer Spectr. 2, pky035 (2018).
Khan, S. S., Singer, B. D. & Vaughan, D. E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16, 624–633 (2017).
Taffett G. E. in Geriatric Medicine: An Evidence-Based Approach, 4th ed. (ed. Cassel C. K.) 27–35 (Springer Science & Business Media, 2003).
Cabeza R., Nyberg L., Park D. C. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging (Oxford Scholarship Online, 2009). https://doi.org/10.1093/acprof:oso/9780195156744.001.0001
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Ganz, P. A. et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J. Natl Cancer Inst. 105, 791–801 (2013).
Bower, J. E. & Ganz, P. A. Symptoms: fatigue and cognitive dysfunction. Adv. Exp. Med. Biol. 862, 53–75 (2015).
Stanton, A. L., Rowland, J. H. & Ganz, P. A. Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am. Psychol. 70, 159–174 (2015).
Chopra, I. & Kamal, K. M. A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual. Life Outcomes 10, 14 (2012).
Howard-Anderson, J., Ganz, P. A., Bower, J. E. & Stanton, A. L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J. Natl Cancer Inst. 104, 386–405 (2012).
Ahles, T. A. & Root, J. C. Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol. 14, 425–451 (2018).
Bernstein, L. J., McCreath, G. A., Komeylian, Z. & Rich, J. B. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci. Biobehav. Rev. 83, 417–428 (2017).
Janelsins, M. C. et al. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin. Oncol. 38, 431–438 (2011).
Lim, A. S. P. et al. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 70, 1544 (2013).
Petrick, J. L. et al. Functional status declines among cancer survivors: trajectory and contributing factors. J. Geriatr. Oncol. 5, 359–367 (2014).
Sehl, M., Lu, X., Silliman, R. & Ganz, P. A. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J. Cancer Surviv. 7, 20–31 (2013).
Arora, M. et al. Longitudinal trajectory of frailty in blood or marrow transplant survivors: report from the blood or marrow transplant survivor study. Cancer 127, 794–800 (2020).
Baker, K. S., Armenian, S. & Bhatia, S. Long-term consequences of hematopoietic stem cell transplantation: current state of the science. Biol. Blood Marrow Transpl. 16 (Suppl. 1), S90–S96 (2010).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Armenian, S. H. et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 118, 1413–1420 (2011).
Ness, K. K. et al. Progression of frailty in young adult survivors of childhood cancer: St. Jude Lifetime Cohort. J. Clin. Oncol. 37, 10057–10057 (2019).
Bennett, J. A., Winters-Stone, K. M., Dobek, J. & Nail, L. M. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol. Nurs. Forum 40, E126–E134 (2013).
Arora, M. et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients. JAMA Oncol. 2, 1277 (2016).
Liu, S. & Kurzrock, R. Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 40, 883–891 (2014).
Totzeck, M., Schuler, M., Stuschke, M., Heusch, G. & Rassaf, T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 280, 163–175 (2019).
Escalante, C. P. et al. Meta-analysis of cardiovascular toxicity risks in cancer patients on selected targeted agents. Support. Care Cancer 24, 4057–4074 (2016).
Joly, F., Castel, H., Tron, L., Lange, M. & Vardy, J. Potential effect of immunotherapy agents on cognitive function in cancer patients. J. Natl Cancer Inst. 112, 123–127 (2020).
Cuzzubbo, S. et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer 73, 1–8 (2017).
Weber, J. S., Yang, J. C., Atkins, M. B. & Disis, M. L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 33, 2092–2099 (2015).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69 (Suppl. 1), S4–S9 (2014).
Salminen, A., Kauppinen, A. & Kaarniranta, K. Emerging role of NF-kB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 24, 835–845 (2012).
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Coppé, J.-P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Freund, A., Orjalo, A. V., Desprez, P.-Y. & Campisi, J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 16, 238–246 (2010).
Erusalimsky, J. D. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 106, 326–332 (2009).
Effros, R. B. The role of CD8 T cell replicative senescence in human aging. Discov. Med. 5, 293–297 (2005).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Yu, E. P. K. & Bennett, M. R. Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol. Metab. 25, 481–487 (2014).
Maassen, J. A. et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 (Suppl. 1), S103–S109 (2004).
Sahin, E. & Depinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010).
Vasto, S. et al. Inflammation, ageing and cancer. Mech Ageing Dev. 130, 40–45 (2009).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Kirkland, J. L., Tchkonia, T., Zhu, Y., Niedernhofer, L. J. & Robbins, P. D. The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 65, 2297–2301 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Palmer, A. K. et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 25, e12950 (2019).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2015).
Elder, S. S. & Emmerson, E. Senescent cells and macrophages: key players for regeneration? Open Biol. 10, 200309 (2020).
Da Silva-Álvarez, S. et al. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 19, e13052 (2020).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Kim, J. H., Jenrow, K. A. & Brown, S. L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 32, 103–115 (2014).
Conroy, S. K. et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res. Treat. 137, 493–502 (2013).
Ermolaeva, M. A. et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501, 416–420 (2013).
Sanoff, H. K. et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl Cancer Inst. 106, dju057 (2014).
Shachar, S. S. et al. Effects of breast cancer adjuvant chemotherapy regimens on expression of the aging biomarker, p16INK4a. JNCI Cancer Spectr. 4, pkaa082 (2020).
Sehl, M. E., Carroll, J. E., Horvath, S. & Bower, J. E. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer 6, 23 (2020).
Scuric, Z. et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer 3, 50 (2017).
Xiao, C. et al. Association of epigenetic age acceleration with risk factors, survival, and quality of life in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 111, 157–167 (2021).
National Cancer Institute. Targeted Cancer Therapies Fact Sheet - National Cancer Institute https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (2020).
Bower, J. E. The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 125, 353–364 (2019).
de Moor, J. S. et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol. Biomark. Prev. 22, 561–570 (2013).
Mandelblatt, J. S. et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J. Clin. Oncol. 3, JCO1800140 (2018).
Bevans, M. et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: the patient-centered outcomes working group report. Biol. Blood Marrow Transpl. 23, 538–551 (2017).
Jim, H. S. L. et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 30, 3578–3587 (2012).
McDougall, G. J., Oliver, J. S. & Scogin, F. Memory and cancer: a review of the literature. Arch. Psychiatr. Nurs. 28, 180–186 (2014).
Epel, E. S. & Lithgow, G. J. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 69 (Suppl. 1), S10–S16 (2014).
Rentscher, K. E., Carroll, J. E. & Mitchell, C. Psychosocial stressors and telomere length: a current review of the science. Annu. Rev. Public Health 41, 223–245 (2020).
Entringer, S., de Punder, K., Buss, C. & Wadhwa, P. D. The fetal programming of telomere biology hypothesis: an update. Philos. Trans. R. Soc. B Biol. Sci. 373, 20170151 (2018).
Carroll, J. E. et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative study. Biol. Psychiatry 81, 136–144 (2017).
Carroll, J. E. et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun. 51, 223–229 (2016).
Carreras, A. et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 37, 1817–1824 (2014).
Schafer, M. J. et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615 (2016).
Quach, A. et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 9, 419–446 (2017).
Rebelo-Marques, A. et al. Aging hallmarks: the benefits of physical exercise. Front. Endocrinol. 9, 258 (2018).
Miller, A. H., Ancoli-Israel, S., Bower, J. E., Capuron, L. & Irwin, M. R. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 26, 971–982 (2008).
Bower, J. E. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 11, 597–609 (2014).
Bower, J. E. et al. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychol. 37, 1025–1034 (2018).
Lazarus R. S. & Folkman S. Stress, Appraisal, and Coping (Springer, 1984).
Cohen S., Kessler R. C. & Gordon L. U. (eds) Measuring Stress: A Guide for Health and Social Scientists (Oxford Univ. Press, 1997).
Carroll, J. E. et al. Childhood abuse, parental warmth, and adult multisystem biological risk in the coronary artery risk development in young adults study. Proc. Natl Acad. Sci. USA 110, 17149–17153 (2013).
Shalev, I. et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18, 576–581 (2013).
Marini, S. et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113, 104484 (2020).
Belsky, D. W. et al. Impact of early personal-history characteristics on the pace of aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16, 644–651 (2017).
Miller, G. E., Chen, E. & Parker, K. J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 137, 959–997 (2011).
Carroll, J. E., Mahrer, N. E., Shalowitz, M., Ramey, S. & Dunkel Schetter, C. Prenatal maternal stress prospectively relates to shorter child buccal cell telomere length. Psychoneuroendocrinology 121, 104841 (2020).
Antoni, M. H. et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248 (2006).
Flint, M. S., Baum, A., Chambers, W. H. & Jenkins, F. J. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32, 470–479 (2007).
Hara, M. R. et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011).
Picard, M., Juster, R.-P. & McEwen, B. S. Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310 (2014).
Epel, E. S. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones 8, 7–22 (2009).
Choi, J., Fauce, S. R. & Effros, R. B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605 (2008).
Picca, A. et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int. J. Mol. Sci. 18, 933 (2017).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Chen, G. Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632 (2011).
Marsland, A. L., Walsh, C., Lockwood, K. & John-Henderson, N. A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 64, 208–219 (2017).
Cohen, S. et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl Acad. Sci. 109, 5995–5999 (2012).
Kiecolt-Glaser, J. K. et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl Acad. Sci. USA 100, 9090–9095 (2003).
Flint, M. S. et al. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. J. Neuroimmunol. 167, 34–44 (2005).
Razzoli, M. et al. Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018).
Mathur, M. B. et al. Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 54, 158–169 (2016).
Shalev, I. et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842 (2013).
Rentscher, K. E. et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a. Psychoneuroendocrinology 102, 139–148 (2019).
Boks, M. P. et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 51, 506–512 (2015).
Simpkin, A. J. et al. Prenatal and early life influences on epigenetic age in children: a study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet. 25, 191–201 (2016).
Roberts, A. L. et al. Posttraumatic stress disorder and accelerated aging: PTSD and leukocyte telomere length in a sample of civilian women. Depress Anxiety 34, 391–400 (2017).
Sumner, J. A., Colich, N. L., Uddin, M., Armstrong, D. & McLaughlin, K. A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry 85, 268–278 (2019).
Zannas, A. S. et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 16, 266 (2015).
Jovanovic, T. et al. Exposure to violence accelerates epigenetic aging in children. Sci. Rep. 7, 8962 (2017).
Marini, S. et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113, 104484 (2020).
Stanton, A. L. Psychosocial concerns and interventions for cancer survivors. J. Clin. Oncol. 24, 5132–5137 (2016).
O’Keefe, E. B., Meltzer, J. P. & Bethea, T. N. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front. Public Health. 3, 51 (2015).
Kumachev, A., Trudeau, M. E. & Chan, K. K. W. Associations among socioeconomic status, patterns of care and outcomes in breast cancer patients in a universal health care system: Ontario’s experience. Cancer 122, 893–898 (2016).
Dignam, J. J. Disparities in breast cancer: narrowing the gap. J. Natl Cancer Inst. 113, 349–350 (2021).
de Punder, K., Heim, C., Wadhwa, P. D. & Entringer, S. Stress and immunosenescence: the role of telomerase. Psychoneuroendocrinology 101, 87–100 (2019).
Cho, H. J., Bower, J. E., Kiefe, C. I., Seeman, T. E. & Irwin, M. R. Early life stress and inflammatory mechanisms of fatigue in the coronary artery risk development in young adults (CARDIA) study. Brain Behav. Immun. 26, 859–865 (2012).
Crosswell, A. D., Bower, J. E. & Ganz, P. A. Childhood adversity and inflammation in breast cancer survivors. Psychosom. Med. 76, 208–214 (2014).
Epel, E. S. & Prather, A. A. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu. Rev. Clin. Psychol. 14, 371–397 (2018).
Han, T. J. et al. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 25, 187–193 (2016).
Bower, J. E., Crosswell, A. D. & Slavich, G. M. Childhood adversity and cumulative life stress: risk factors for cancer-related fatigue. Clin. Psychol. Sci. 2, 108–115 (2014).
Bower, J. E. et al. Childhood maltreatment and monocyte gene expression among women with breast cancer. Brain Behav. Immun. 88, 396–402 (2020).
Bower, J. E. et al. Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125, 633–641 (2019).
Papanastasiou, A. et al. Role of stress, age and adjuvant therapy in the cognitive function of patients with breast cancer. Oncol. Lett. 18, 507–517 (2019).
Huehnchen, P., van Kampen, A., Boehmerle, W. & Endres, M. Cognitive impairment after cytotoxic chemotherapy. Neurooncol. Pract. 7, 11–21 (2020).
Kuring, J. K., Mathias, J. L. & Ward, L. Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: a systematic review and meta-analysis. J. Affect. Disord. 274, 247–261 (2020).
Watson, N. F. et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38, 1161–1183 (2015).
Carroll, J. E. & Prather, A. A. Sleep and biological aging: a short review. Curr. Opin. Endocr. Metab. Res. 18, 159–164 (2021).
Brown, M. K. & Naidoo, N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol. Neurobiol. 42, 103–113 (2010).
Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: a beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Tempaku, P. F., Mazzotti, D. R. & Tufik, S. Telomere length as a marker of sleep loss and sleep disturbances: a potential link between sleep and cellular senescence. Sleep Med. 16, 559–563 (2015).
Prather, A. A. et al. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun. 47, 155–162 (2015).
Cribbet, M. R. et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 37, 65–70 (2014).
Jackowska, M. et al. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II Cohort Study. PLoS ONE 7, e47292 (2012).
Huang, P. et al. The association between obstructive sleep apnea and shortened telomere length: a systematic review and meta-analysis. Sleep Med. 48, 107–112 (2018).
Prather, A. A. et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J. Aging Res. 2011, 1–6 (2011).
Lee, K. A. et al. Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. Sleep 37, 157–166 (2014).
Zgheib, N. K. et al. Short telomere length is associated with aging, central obesity, poor sleep and hypertension in lebanese individuals. Aging Dis. 9, 77–89 (2018).
Carroll, J. E. et al. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the multi-ethnic study of atherosclerosis. Sleep 42, zsz089 (2019).
Carroll, J. E. et al. Insomnia and telomere length in older adults. Sleep 39, 559–564 (2016).
Irwin, M. R. & Opp, M. R. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155 (2017).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52 (2016).
Garland, S. N., Mahon, K. & Irwin, M. R. Integrative approaches for sleep health in cancer survivors. Cancer J. 25, 337–342 (2019).
Palesh, O. et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 37, 837–842 (2014).
Trudel-Fitzgerald, C. et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 116, 1239–1246 (2017).
Irwin, M. R., Olmstead, R. E., Ganz, P. A. & Haque, R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav. Immun. 30 (Suppl.), S58–S67 (2013).
Savard, J. & Morin, C. M. Insomnia in the context of cancer: a review of a neglected problem. J. Clin. Oncol. 19, 895–908 (2001).
Carroll, J. E. et al. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: interaction with genotype. Cancer 125, 4516–4524 (2019).
Ancoli-Israel, S. et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support. Care Cancer 22, 2535–2545 (2014).
Savard, J. et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep 32, 1155–1160 (2009).
Santoso, A. M. M. et al. Prevalence of sleep disturbances among head and neck cancer patients: a systematic review and meta-analysis. Sleep Med. Rev. 47, 62–73 (2019).
Basen-Engquist, K. et al. Agenda for translating physical activity, nutrition, and weight management interventions for cancer survivors into clinical and community practice. Obesity 25 (Suppl. 2), S9–S22 (2017).
Tucker, L. A. Physical activity and telomere length in U.S. men and women: an NHANES investigation. Prev. Med. 100, 145–151 (2017).
Cherkas, L. F. et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 168, 154–158 (2008).
Soares-Miranda, L. et al. Physical activity, physical fitness, and leukocyte telomere length. Med. Sci. Sports Exerc. 47, 2525–2534 (2015).
Puterman, E. & et al. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: a randomized controlled trial - Curt Richter Award Paper 2018. Psychoneuroendocrinology 98, 245–252 (2018).
Figueiredo, N. et al. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 39, 874–884 (2013).
Garatachea, N. et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 18, 57–89 (2015).
Ramírez-Vélez, R. et al. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers 13, 264 (2021).
Speck, R. M., Courneya, K. S., Mâsse, L. C., Duval, S. & Schmitz, K. H. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J. Cancer Surviv. 4, 87–100 (2010).
Englund, D. A. et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20, e13415 (2021).
Kerr, J., Anderson, C. & Lippman, S. M. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 18, e457–e471 (2017).
Arem, H. et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the national institutes of health-AARP diet and health study. J. Clin. Oncol. 33, 180–188 (2015).
Ligibel, J. A., Basen-Engquist, K. & Bea, J. W. Weight management and physical activity for breast cancer prevention and control. Am. Soc. Clin. Oncol. Educ. B. 39, e22–e33 (2019).
Wagner, M. A., Erickson, K. I., Bender, C. M. & Conley, Y. P. The influence of physical activity and epigenomics on cognitive function and brain health in breast cancer. Front. Aging Neurosci. 12, 123 (2020).
Bender, C. M. et al. Physical activity, cardiorespiratory fitness, and cognitive function in postmenopausal women with breast cancer. Support. Care Cancer 29, 3743–3752 (2020).
Ho, M. et al. Effects of dietary and physical activity interventions on generic and cancer-specific health-related quality of life, anxiety, and depression in colorectal cancer survivors: a randomized controlled trial. J. Cancer Surviv. 14, 424–433 (2020).
Thorsen, L. et al. The association between self-reported physical activity and prevalence of depression and anxiety disorder in long-term survivors of testicular cancer and men in a general population sample. Support. Care Cancer 13, 637–646 (2005).
Ribeiro, F. E. et al. Relationship of anxiety and depression symptoms with the different domains of physical activity in breast cancer survivors. J. Affect. Disord. 273, 210–214 (2020).
An, R. & Yan, H. Body weight status and telomere length in U.S. middle-aged and older adults. Obes. Res. Clin. Pract. 11, 51–62 (2017).
Hang, D. et al. Longitudinal associations of lifetime adiposity with leukocyte telomere length and mitochondrial DNA copy number. Eur. J. Epidemiol. 33, 485–495 (2018).
Horvath, S. et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014).
Nevalainen, T. et al. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin. Epigenetics 9, 20 (2017).
Ogrodnik, M. et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077.e8 (2019).
Almendáriz-Palacios, C., Mousseau, D. D., Eskiw, C. H. & Gillespie, Z. E. Still living better through chemistry: an update on caloric restriction and caloric restriction mimetics as tools to promote health and lifespan. Int. J. Mol. Sci. 21, 9220 (2020).
Hoshino, S., Kobayashi, M. & Higami, Y. Mechanisms of the anti-aging and prolongevity effects of caloric restriction: evidence from studies of genetically modified animals. Aging 10, 2243 (2018).
Dorling, J. L. et al. Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2. Nutr. Rev. 79, 98–113 (2021).
Gonzalez-Freire, M. et al. The road ahead for health and lifespan interventions. Ageing Res. Rev. 59, 101037 (2020).
Del Bo’, C. D. et al. Overview of human intervention studies evaluating the impact of the mediterranean diet on markers of DNA damage. Nutrients 11, 391 (2019).
Sung, H., Hyun, N., Leach, C. R., Yabroff, K. R. & Jemal, A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. J. Am. Med. Assoc. 324, 2521–2535 (2020).
Ganz, P. A. & Casillas, J. N. Incorporating the risk for subsequent primary cancers into the care of adult cancer survivors: moving beyond 5-year survival. J. Am. Med. Assoc. 324, 2493–2495 (2020).
Ruiz-Casado, A., Álvarez-Bustos, A., de Pedro, C. G., Méndez-Otero, M. & Romero-Elías, M. Cancer-related fatigue in breast cancer survivors: a review. Clin. Breast Cancer 21, 10–25 (2021).
Lee, J. T. et al. Impact of community-based exercise program participation on aerobic capacity in women with and without breast cancer. World J. Clin. Oncol. 12, 468–481 (2021).
Hamajima, N. et al. Alcohol, tobacco and breast cancer - collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br. J. Cancer 87, 1234–1245 (2002).
Znaor, A. et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int. J. Cancer 105, 681–686 (2003).
Baan, R. et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 8, 292–293 (2007).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Advances in Knowledge of the Health Consequences of Smoking: From 1964–2014, https://www.ncbi.nlm.nih.gov/books/NBK294317/ (2014).
Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General https://pubmed.ncbi.nlm.nih.gov/21452462/ (2020).
Harpaz, T. et al. The effect of ethanol on telomere dynamics and regulation in human cells. Cells 7, 169 (2018).
Beach, S. R. H. et al. Methylomic aging as a window onto the influence of lifestyle: tobacco and alcohol use alter the rate of biological aging. J. Am. Geriatr. Soc. 63, 2519–2525 (2015).
Dixit, S. et al. Alcohol consumption and leukocyte telomere length. Sci. Rep. 9, 1404 (2019).
Latifovic, L., Peacock, S. D., Massey, T. E. & King, W. D. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol. Biomark. Prev. 25, 374–380 (2016).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019).
Lei, M.-K., Gibbons, F. X., Simons, R. L., Philibert, R. A. & Beach, S. R. H. The effect of tobacco smoking differs across indices of DNA methylation-based aging in an African American sample: DNA methylation-based indices of smoking capture these effects. Genes 11, 311 (2020).
Verde, Z. et al. Effects of cigarette smoking and nicotine metabolite ratio on leukocyte telomere length. Environ. Res. 140, 488–494 (2015).
Needham, B. L. et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc. Sci. Med. 85, 1–8 (2013).
Valdes, A. et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005).
Collins, F. Connecting senescent cells to obesity and anxiety. NIH Director’s Blog, https://directorsblog.nih.gov/2019/01/08/connecting-senescent-cells-to-obesity-and-anxiety/ (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02652052 (2019).
Hiller, J. G., Perry, N. J., Poulogiannis, G., Riedel, B. & Sloan, E. K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15, 205–218 (2017).
Hiller, J. G. et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res. 26, 1803–1811 (2020).
Knight, J. M. et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 4, 467–476 (2020).
Haldar, R. et al. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer 126, 3991–4001 (2020).
Cole, S. W. & Sood, A. K. Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res. 18, 1201–1206 (2012).
Carlson, L. E., Toivonen, K. & Subnis, U. Integrative approaches to stress management. Cancer J. 25, 329–336 (2019).
Greenlee, H. et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin. 67, 194–232 (2017).
Matis, J., Svetlak, M., Slezackova, A., Svoboda, M. & Šumec, R. Mindfulness-based programs for patients with cancer via eHealth and mobile health: systematic review and synthesis of quantitative research. J. Med. Internet Res. 22, e20709 (2020).
Antoni, M. H. et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiatry 71, 366–372 (2012).
Antoni, M. H. et al. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology 74, 269–277 (2016).
Bower, J. E. et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer 121, 1231–1240 (2015).
Bower, J. E. & Irwin, M. R. Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav. Immun. 51, 1–11 (2016).
Chaix, R. et al. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology 85, 210–214 (2017).
Bower, J. E. et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology 43, 20–29 (2014).
Kiecolt-Glaser, J. K. et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol. 32, 1040–1049 (2014).
Danhauer, S. C. et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer 125, 1979–1989 (2019).
Garland, S. N. et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat. 10, 1113–1124 (2014).
Johnson, J. A. et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med. Rev. 27, 20–28 (2016).
Zachariae, R. et al. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J. Natl Cancer Inst. 110, 880–887 (2018).
Irwin, M. R. et al. Tai Chi Chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast cancer: a randomized, partially blinded, noninferiority trial. J. Clin. Oncol. 35, 2656–2665 (2017).
Garland, S. N. et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J. Clin. Oncol. 32, 449–457 (2014).
Zhao, Y. et al. Effects of mindfulness-based cognitive therapy on breast cancer survivors with insomnia: a randomised controlled trial. Eur. J. Cancer Care 29, e13259 (2020).
Mustian, K. M. et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J. Clin. Oncol. 31, 3233–3241 (2013).
Garland, S. N. et al. Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial. J. Natl Cancer Inst. 111, 1323–1331 (2019).
Irwin, M. R. et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep 37, 1543–1552 (2014).
Irwin, M. R. et al. Tai Chi, cellular Inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. JNCI Monogr. 2014, 295–301 (2014).
Chen, T.-Y., Lee, S. & Buxton, O. M. A greater extent of insomnia symptoms and physician-recommended sleep medication use predict fall risk in community-dwelling older adults. Sleep https://doi.org/10.1093/sleep/zsx142 (2017).
Besedovsky, L. et al. Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat. Commun. 8, 1984 (2017).
Charalambous, A. et al. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support. Care Cancer 27, 2747–2753 (2019).
Matthews, E., Carter, P., Page, M., Dean, G. & Berger, A. Sleep-wake disturbance: a systematic review of evidence-based interventions for management in patients with cancer. Clin. J. Oncol. Nurs. 22, 37–52 (2018).
Piercy, K. L. et al. The physical activity guidelines for Americans. J. Am. Med. Assoc. 320, 2020–2028 (2018).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 129 (Suppl. 2), S102–S138 (2014).
Kaiser, E. G., Prochaska, J. J. & Kendra, M. S. Tobacco cessation in oncology care. Oncology 95, 129–137 (2018).
Warren, G. W., Ostroff, J. S. & Goffin, J. R. Lung cancer screening, cancer treatment, and addressing the continuum of health risks caused by tobacco. Am. Soc. Clin. Oncol. Educ. B. 35, 223–229 (2016).
LoConte, N. K. et al. Lifestyle modifications and policy implications for primary and secondary cancer prevention: diet, exercise, sun safety, and alcohol reduction. Am. Soc. Clin. Oncol. Educ. B. 38, 88–100 (2018).
Demark-Wahnefried, W. et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin. 68, 64–89 (2018).
Ligibel, J. A. et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: Results from the pre-operative health and body (PreHAB) study. Clin. Cancer Res. 25, 5398–5406 (2019).
Gerritsen, J. K. W. & Vincent, A. J. P. E. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 50, 796–803 (2016).
Ballard-Barbash, R. et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J. Natl Cancer Inst. 104, 815–840 (2012).
García-Calzón, S. et al. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PLoS ONE 9, e89828 (2014).
Mctiernan, A. et al. Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc. 51, 1252–1261 (2019).
Pinckard, K., Baskin, K. K. & Stanford, K. I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 6, 69 (2019).
Brown, J. C. et al. Effect of exercise or metformin on biomarkers of inflammation in breast and colorectal cancer: a randomized trial. Cancer Prev. Res. 13, 1055–1062 (2020).
Bower, J. E. et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J. Clin. Oncol. 32, 1840–1850 (2014).
Pekmezi, D. et al. Physical activity maintenance following home-based, individually tailored print interventions for African American women. Health Promot. Pract. 21, 268–276 (2020).
Mayer, D. K. et al. SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? J. Cancer Surviv. 12, 82–94 (2018).
Demark-Wahnefried, W. et al. Pilot randomized controlled trial of a home vegetable gardening intervention among older cancer survivors shows feasibility, satisfaction, and promise in improving vegetable and fruit consumption, reassurance of worth, and the trajectory of central adiposity. J. Acad. Nutr. Diet. 118, 689–704 (2018).
Guida, J. L. et al. Strategies to prevent or remediate cancer and treatment-related aging. J. Natl Cancer Inst. 113, 112–122 (2021).
Brandhorst, S. et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 (2015).
Longo, V. D. & Mattson, M. P. Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014).
Mattson, M. P., Longo, V. D. & Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 39, 46–58 (2017).
De Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Hahn, O. et al. A nutritional memory effect counteracts the benefits of dietary restriction in old mice. Nat. Metab. 1, 1059–1073 (2019).
Espada, L. et al. Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat. Metab. 2, 1316–1331 (2020).
Fucito, L. M. et al. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the association for the treatment of tobacco use and dependence and the society for research on nicotine and tobacco. Cancer 122, 1150–1159 (2016).
Taylor, K. L. et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: a randomized clinical trial. Lung Cancer 108, 242–246 (2017).
Zbikowski, S. M., Hapgood, J., Barnwell, S. S. & McAfee, T. Phone and web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J. Med. Internet Res. 10, e41 (2008).
McHugh, D. & Gil, J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018).
Van Waart, H. et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J. Clin. Oncol. 33, 1918–1927 (2015).
Courneya, K. S. et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 46, 1744–1751 (2014).
Cannioto, R. A. et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: relationships with survival. J. Natl Cancer Inst. 113, 54–63 (2021).
Antoni, M. H. et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am. J. Psychiatry 163, 1791–1797 (2006).
Gudenkauf, L. M. et al. Brief cognitive-behavioral and relaxation training interventions for breast cancer: a randomized controlled trial. J. Consult. Clin. Psychol. 83, 677–688 (2015).
Palesh, O. et al. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. Br. J. Cancer 119, 274–281 (2018).
Johnson, J. A. et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J. Cancer Surviv. 12, 206–215 (2018).
Redd, W. H. et al. Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology 23, 1431–1434 (2014).
Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019).
Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020).
Savard, J., Ivers, H., Savard, M.-H. & Morin, C. M. Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer 121, 1703–1711 (2015).
Palesh, O. et al. Secondary outcomes of a behavioral sleep intervention: a randomized clinical trial. Health Psychol. 38, 196–205 (2019).
Carlson, L., Rouleau, C. R. & Garland, S. N. The impact of mindfulness-based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag. Res. 7, 121 (2015).
Blackburn, E. H. Telomere states and cell fates. Nature 408, 53–56 (2000).
Rodriguez–Mortera, R., Bains, Y. & Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. 24, 186–211 (2019).
Nowotny, B. et al. Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm. Metab. Res. 42, 746–753 (2010).
Fiorentino, T. V., Prioletta, A., Zuo, P. & Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 19, 5695–5703 (2013).
Acknowledgements
The work of J.E.C. was supported in part by the American Cancer Society Research Scholars grant 128660-RSG-15-187-01-PCSM and the National Cancer Institute at the National Institutes of Health grant R01CA237535 and R35CA197289. The work of J.E.B. was supported in part by the National Cancer Institute at the National Institutes of Health grant R01CA237535 and the Breast Cancer Research Foundation. The work of P.A.G. was supported by the Breast Cancer Research Foundation and the author notes that she serves on the Scientific Advisory Board of the Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Clinical Oncology thanks K. Ness and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Rights and permissions
About this article
Cite this article
Carroll, J.E., Bower, J.E. & Ganz, P.A. Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care. Nat Rev Clin Oncol 19, 173–187 (2022). https://doi.org/10.1038/s41571-021-00580-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00580-3
This article is cited by
-
Aging and cancer
Molecular Cancer (2024)
-
Disparities, aging and childhood cancer
Nature Cancer (2024)
-
Combined aerobic and strength exercise training on biological ageing in Singaporean breast cancer patients: protocol for the Breast Cancer Exercise Intervention (BREXINT) Pilot Study
GeroScience (2024)
-
Health-related conditions among long-term cancer survivors diagnosed in adolescence and young adulthood (AYA): results of the SURVAYA study
Journal of Cancer Survivorship (2024)
-
A scoping review evaluating physical and cognitive functional outcomes in cancer survivors treated with chemotherapy: charting progress since the 2018 NCI think tank on cancer and aging phenotypes
Journal of Cancer Survivorship (2024)