Abstract
Nine different antibody–drug conjugates (ADCs) are currently approved as cancer treatments, with dozens more in preclinical and clinical development. The primary goal of ADCs is to improve the therapeutic index of antineoplastic agents by restricting their systemic delivery to cells that express the target antigen of interest. Advances in synthetic biochemistry have ushered in a new generation of ADCs, which promise to improve upon the tissue specificity and cytotoxicity of their predecessors. Many of these drugs have impressive activity against treatment-refractory cancers, although hurdles impeding their broader use remain, including systemic toxicity, inadequate biomarkers for patient selection, acquired resistance and unknown benefit in combination with other cancer therapies. Emerging evidence indicates that the efficacy of a given ADC depends on the intricacies of how the antibody, linker and payload components interact with the tumour and its microenvironment, all of which have important clinical implications. In this Review, we discuss the current state of knowledge regarding the design, mechanism of action and clinical efficacy of ADCs as well as the apparent limitations of this treatment class. We then propose a path forward by highlighting several hypotheses and novel strategies to maximize the potential benefit that ADCs can provide to patients with cancer.
Key points
-
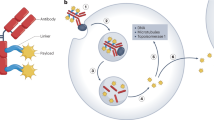
Antibody–drug conjugates (ADCs) comprise three main components: an antibody, a linker and a payload. The clinical properties of ADCs depend on the characteristics of all three of these components.
-
The mechanism of action of ADCs is complex, often requiring drug internalization followed by intracellular processing and payload release. Unlike many standard therapies used in oncology, ADCs must be acted upon by cancer cells for optimal effectiveness.
-
The pharmacodynamic properties of ADCs make them uniquely suited for activity in treatment-refractory cancers, which is reflected in the current clinical indications for ADCs in oncology.
-
ADCs exhibit both on-target and off-target toxicities; while most toxicities seem to be related to the nature of the payload, notable examples of target-dependent toxicities exist.
-
Important and potentially practice-changing innovations in ADC design, biomarker development and combination therapies are ongoing in preclinical and clinical studies.
-
An improved understanding of the interactions between ADCs and tumours is essential for clinicians and scientists to realize the true potential of this drug class for the treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tolcher, A. W. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann. Oncol. 27, 2168–2172 (2016).
Alley, S. C., Okeley, N. M. & Senter, P. D. Antibody-drug conjugates: targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 14, 529–537 (2010).
Carter, P. J. & Senter, P. D. Antibody-drug conjugates for cancer therapy. Cancer J. 14, 154–169 (2008).
Sievers, E. L. & Senter, P. D. Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 64, 15–29 (2013).
Drake, P. M. & Rabuka, D. Recent developments in ADC technology: preclinical studies signal future clinical trends. BioDrugs 31, 521–531 (2017).
Deonarain, M. P., Yahioglu, G., Stamati, I. & Marklew, J. Emerging formats for next-generation antibody drug conjugates. Expert Opin. Drug Dis. 10, 463–481 (2015).
Beck, A., Goetsch, L., Dumontet, C. & Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Ehrlich, P. in The Collected Papers of Paul Ehrlich 596-618 (Pergamon, 1956).
Strebhardt, K. & Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 8, 473–480 (2008).
Mathe, G., Lou, T. B. & Bernard, J. Effet sur la leucemie 1210 de la souris dune combinaison par diazotation da-methopterine et de gamma-globulines de hamsters porteurs de cette leucemie par heterogreffe. Presse Med. 66, 571–571 (1958).
Rowland, G. F., Oneill, G. J. & Davies, D. A. L. Suppression of tumor-growth in mice by a drug-antibody conjugate using a novel approach to linkage. Nature 255, 487–488 (1975).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Moolten, F. L. & Cooperband, S. R. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science 169, 68–70 (1970).
Elias, D. J. et al. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunconjugate in patients with non-small cell lung carcinoma. Cancer Res. 50, 4154–4159 (1990).
Saleh, M. N. et al. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-Doxorubicin in patients with Lewis Y-expressing epithelial tumors. J. Clin. Oncol. 18, 2282–2292 (2000).
Schneck, D. et al. Disposition of a murine monoclonal antibody vinca conjugate (KS1/4-DAVLB) in patients with adenocarcinomas. Clin. Pharmacol. Ther. 47, 36–41 (1990).
Ford, C. H. et al. Localisation and toxicity study of a vindesine-anti-CEA conjugate in patients with advanced cancer. Br. J. Cancer 47, 35–42 (1983).
Sievers, E. L. et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 19, 3244–3254 (2001).
Bross, P. F. et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 7, 1490–1496 (2001).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 30, 2183–2189 (2012).
Younes, A., Yasothan, U. & Kirkpatrick, P. Brentuximab vedotin. Nat. Rev. Drug Discov. 11, 19–20 (2012).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 17, 197–223 (2018).
Schuurman, J. & Parren, P. W. Editorial overview: special section: new concepts in antibody therapeutics: what’s in store for antibody therapy? Curr. Opin. Immunol. 40, Vii–Xiii (2016).
Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. https://doi.org/10.3389/fimmu.2014.00520 (2014).
Tiller, K. E. & Tessier, P. M. Advances in antibody design. Annu. Rev. Biomed. Eng. 17, 191–216 (2015).
Yu, J. F., Song, Y. P. & Tian, W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 13, 45 (2020).
Agarwal, P. & Bertozzi, C. R. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 26, 176–192 (2015).
Hoffmann, R. M. et al. Antibody structure and engineering considerations for the design and function of antibody drug conjugates (ADCs). Oncoimmunology 7, e1395127 (2018).
Hock, M. B., Thudium, K. E., Carrasco-Triguero, M. & Schwabe, N. F. Immunogenicity of Antibody drug conjugates: bioanalytical methods and monitoring strategy for a novel therapeutic modality. AAPS J. 17, 35–43 (2015).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Bardia, A. et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 380, 741–751 (2019).
Rosenberg, J. E. et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 37, 2592–2600 (2019).
Stepan, L. P. et al. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J. Histochem. Cytochem. 59, 701–710 (2011).
Pegram, M. D., Konecny, G. & Slamon, D. J. The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer. Cancer Treat. Res. 103, 57–75 (2000).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
van der Weyden, C. A., Pileri, S. A., Feldman, A. L., Whisstock, J. & Prince, H. M. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 7, e603 (2017).
Tedder, T. F., Tuscano, J., Sato, S. & Kehrl, J. H. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu. Rev. Immunol. 15, 481–504 (1997).
Pfeifer, M. et al. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 29, 1578–1586 (2015).
Gebhart, G. et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann. Oncol. 27, 619–624 (2016).
Metzger, O. et al. HER2 heterogeneity as a predictor of response to neoadjuvant T-DM1 plus pertuzumab: results from a prospective clinical trial. J. Clin. Oncol. 37 (Suppl. 15), 502 (2019).
de Goeij, B. E. et al. High turnover of tissue factor enables efficient intracellular delivery of antibody-drug conjugates. Mol. Cancer Ther. 14, 1130–1140 (2015).
Damelin, M., Zhong, W. Y., Myers, J. & Sapra, P. Evolving strategies for target selection for antibody-drug conjugates. Pharm. Res. 32, 3494–3507 (2015).
Jain, N., Smith, S. W., Ghone, S. & Tomczuk, B. Current ADC linker chemistry. Pharm. Res. 32, 3526–3540 (2015).
Tsuchikama, K. & An, Z. Q. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell 9, 33–46 (2018).
Drake, P. M. & Rabuka, D. An emerging playbook for antibody-drug conjugates: lessons from the laboratory and clinic suggest a strategy for improving efficacy and safety. Curr. Opin. Chem. Biol. 28, 174–180 (2015).
Hamann, P. R. et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 13, 47–58 (2002).
Erickson, H. K. et al. The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Mol. Cancer Ther. 11, 1133–1142 (2012).
Goldenberg, D. M., Cardillo, T. M., Govindan, S. V., Rossi, E. A. & Sharkey, R. M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6, 22496–22512 (2015).
Polson, A. G. et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res. 69, 2358–2364 (2009).
Kanellos, J., Pietersz, G. A. & Mckenzie, I. F. Studies of methotrexate monoclonal-antibody conjugates for immunotherapy. J. Natl Cancer Inst. 75, 319–332 (1985).
Starling, J. J. et al. In vivo antitumor activity of a monoclonal antibody-Vinca alkaloid immunoconjugate directed against a solid tumor membrane antigen characterized by heterogeneous expression and noninternalization of antibody-antigen complexes. Cancer Res. 51, 2965–2972 (1991).
Trail, P. A. et al. Cure of xenografted human carcinomas by Br96-doxorubicin immunoconjugates. Science 261, 212–215 (1993).
Teicher, B. A. & Chari, R. V. J. Antibody conjugate therapeutics: challenges and potential. Clin. Cancer Res. 17, 6389–6397 (2011).
Sedlacek, H. H. Antibodies as Carriers of Cytotoxicity (Karger, 1992).
Mach, J.-P. et al. Tumor localization of radio-labeled antibodies against carcinoembryonic antigen in patients with carcinoma: a critical evaluation. N. Engl. J. Med. 303, 5–10 (1980).
Liu, C. N. et al. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc. Natl Acad. Sci. USA 93, 8618–8623 (1996).
Senter, P. D. Potent antibody drug conjugates for cancer therapy. Curr. Opin. Chem. Biol. 13, 235–244 (2009).
Waight, A. B. et al. Structural basis of microtubule destabilization by potent auristatin anti-mitotics. PLoS ONE 11, e0160890 (2016).
Ricart, A. D. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin. Cancer Res. 17, 6417–6427 (2011).
Oroudjev, E. et al. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol. Cancer Ther. 9, 2700–2713 (2010).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Hamblett, K. J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Sun, X. X. et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjugate Chem. 28, 1371–1381 (2017).
Lyon, R. P. et al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 33, 733–735 (2015).
Ogitani, Y., Hagihara, K., Oitate, M., Naito, H. & Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 107, 1039–1046 (2016).
Li, F. et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 76, 2710–2719 (2016).
Tolcher, A. W. The evolution of antibody-drug conjugates: a positive inflexion point. Am. Soc. Clin. Oncol. Educ. Book 40, 1–8 (2020).
Girish, S. et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother. Pharmacol. 69, 1229–1240 (2012).
Bender, B. C. et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother. Pharmacol. 70, 591–601 (2012).
Singh, A. P. & Shah, D. K. Application of a PK-PD modeling and simulation-based strategy for clinical translation of antibody-drug conjugates: a case study with trastuzumab emtansine (T-DM1). AAPS J. 19, 1054–1070 (2017).
Fujimori, K., Covell, D. G., Fletcher, J. E. & Weinstein, J. N. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J. Nucl. Med. 31, 1191–1198 (1990).
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006).
Matsumura, Y. Cancer stromal targeting therapy to overcome the pitfall of EPR effect. Adv. Drug Deliv. Rev. 154-155, 142–150 (2020).
Thurber, G. M. & Wittrup, K. D. A mechanistic compartmental model for total antibody uptake in tumors. J. Theor. Biol. 314, 57–68 (2012).
Chari, R. V. J. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc. Chem. Res. 41, 98–107 (2008).
Lu, G. et al. Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat. Commun. 11, 5667 (2020).
Alley, S. C. et al. The pharmacologic basis for antibody-auristatin conjugate activity. J. Pharmacol. Exp. Ther. 330, 932–938 (2009).
Giddabasappa, A. et al. Biodistribution and targeting of Anti-5T4 antibody-drug conjugate using fluorescence molecular tomography. Mol. Cancer Ther. 15, 2530–2540 (2016).
Juweid, M. et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 52, 5144–5153 (1992).
Ritchie, M., Tchistiakova, L. & Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 5, 13–21 (2013).
Acchione, M., Kwon, H., Jochheim, C. M. & Atkins, W. M. Impact of linker and conjugation chemistry on antigen binding, Fc receptor binding and thermal stability of model antibody-drug conjugates. mAbs 4, 362–372 (2012).
Redman, J. M., Hill, E. M., AlDeghaither, D. & Weiner, L. M. Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 67, 28–45 (2015).
Junttila, T. T., Li, G. M., Parsons, K., Phillips, G. L. & Sliwkowski, M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 128, 347–356 (2011).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Tai, Y. T. et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 123, 3128–3138 (2014).
Kovtun, Y. V. & Goldmacher, V. S. Cell killing by antibody-drug conjugates. Cancer Lett. 255, 232–240 (2007).
Jedema, I. et al. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia 18, 316–325 (2004).
Sutherland, M. S. K. et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J. Biol. Chem. 281, 10540–10547 (2006).
Erickson, H. K. et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 66, 4426–4433 (2006).
Staudacher, A. H. & Brown, M. P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Brit. J. Cancer 117, 1736–1742 (2017).
Kovtun, Y. V. et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66, 3214–3221 (2006).
Dokter, W. et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 14, 692–7030 (2015).
Singh, A. P., Sharma, S. & Shah, D. K. Quantitative characterization of in vitro bystander effect of antibody-drug conjugates. J. Pharmacokinet. Pharmacodyn. 43, 567–582 (2016).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Drakaki, A. et al. Docetaxel with or without ramucirumab after immune checkpoint inhibition in platinum-refractory metastatic urothelial carcinoma (mUC): Prespecified subgroup analysis from the phase 3 RANGE trial. J. Clin. Oncol. 36 (Suppl. 6), 434 (2018).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Barok, M., Tanner, M., Koninki, K. & Isola, J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 13, R46 (2011).
Coley, H. M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 34, 378–390 (2008).
Perez, E. A. et al. Randomized phase II study of two irinotecan schedules for patients with metastatic breast cancer refractory to an anthracycline, a taxane, or both. J. Clin. Oncol. 22, 2849–2855 (2004).
National Comprehensive Cancer Network. Breast cancer https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2021).
Seol, H. et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod. Pathol. 25, 938–948 (2012).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a Phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020).
Tijink, B. M. et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin. Cancer Res. 12, 6064–6072 (2006).
Kerckhove, N. et al. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front. Pharmacol. 8, 86 (2017).
Kamba, T. & McDonald, D. M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Brit. J. Cancer 96, 1788–1795 (2007).
Sorrentino, M. F., Kim, J., Foderaro, A. E. & Truesdell, A. G. 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol. J. 19, 453–458 (2012).
Sakamoto, S. et al. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 49, 745–752 (1989).
Tolcher, A. W. et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J. Clin. Oncol. 17, 478–484 (1999).
Riechelmann, H. et al. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral. Oncol. 44, 823–829 (2008).
Donaghy, H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. mAbs 8, 659–671 (2016).
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1124–1135 (2019).
Sendur, M. A., Aksoy, S. & Altundag, K. Cardiotoxicity of novel HER2-targeted therapies. Curr. Med. Res. Opin. 29, 1015–1024 (2013).
Ponde, N. et al. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur. J. Cancer 126, 65–73 (2020).
Cote, G. M., Sawyer, D. B. & Chabner, B. A. ERBB2 inhibition and heart failure. N. Engl. J. Med. 367, 2150–2153 (2012).
Saber, H. & Leighton, J. K. An FDA oncology analysis of antibody-drug conjugates. Regul. Toxicol. Pharm. 71, 444–452 (2015).
Masters, J. C., Nickens, D. J., Xuan, D., Shazer, R. L. & Amantea, M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest. New Drugs 36, 121–135 (2018).
Eaton, J. S., Miller, P. E., Mannis, M. J. & Murphy, C. J. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J. Ocul. Pharmacol. Ther. 31, 589–604 (2015).
de Goeij, B. E. & Lambert, J. M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 40, 14–23 (2016).
Bardia, A. et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 35, 2141–2148 (2017).
Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P. & Goldenberg, D. M. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 17, 3157–3169 (2011).
Mahalingaiah, P. K. et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol. Ther. 200, 110–125 (2019).
Uppal, H. et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin. Cancer Res. 21, 123–133 (2015).
Zhao, H. et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 78, 2115–2126 (2018).
Makawita, S. & Meric-Bernstam, F. Antibody-drug conjugates: patient and treatment selection. Am. Soc. Clin. Oncol. Educ. Book 40, 1–10 (2020).
Ott, P. A. et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J. Clin. Oncol. 32, 3659–3666 (2014).
Yardley, D. A. et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J. Clin. Oncol. 33, 1609–1619 (2015).
Saber, H., Simpson, N., Ricks, T. K. & Leighton, J. K. An FDA oncology analysis of toxicities associated with PBD-containing antibody-drug conjugates. Regul. Toxicol. Pharm. 107, 104429 (2019).
Drago, J. Z. et al. Inferences about drug safety in phase 3 trials in oncology: Examples from advanced prostate cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa134 (2020).
Yardley, D. A. et al. Quantitative measurement of HER2 expression in breast cancers: comparison with ‘real-world’ routine HER2 testing in a multicenter collaborative biomarker Study and correlation with overall survival. Breast Cancer Res. 17, 41 (2015).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 142, 1364–1382 (2018).
Li, B. T. et al. Ado-trastuzumab emtansine for patients With HER2-mutant lung cancers: results from a phase II basket trial. J. Clin. Oncol. 36, 2532–2537 (2018).
Tarantino, P. et al. HER2-low breast cancer: pathological and clinical landscape. J. Clin. Oncol. 38, 1951–1962 (2020).
Hurvitz, S. A. et al. Biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. https://www.abstractsonline.com/pp8/#!/9223/presentation/674 (2020).
Gainor, J. F. & Shaw, A. T. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J. Clin. Oncol. 31, 3987–3996 (2013).
Loganzo, F., Sung, M. & Gerber, H. P. Mechanisms of resistance to antibody-drug conjugates. Mol. Cancer Ther. 15, 2825–2834 (2016).
Loganzo, F. et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol. Cancer Ther. 14, 952–963 (2015).
Li, G. M. et al. Mechanisms of acquired resistance to trastuzumab emtansine in breast cancer cells. Mol. Cancer Ther. 17, 1441–1453 (2018).
Rios-Luci, C. et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. 77, 4639–4651 (2017).
Chen, R. et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol. Cancer Ther. 14, 1376–1384 (2015).
Walter, R. B. et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 109, 4168–4170 (2007).
Szakacs, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C. & Gottesman, M. M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 (2006).
Jackson, D. & Stover, D. Using the lessons learned from the clinic to improve the preclinical development of antibody drug conjugates. Pharm. Res. 32, 3458–3469 (2015).
Takegawa, N. et al. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 141, 1682–1689 (2017).
Chandarlapaty, S. et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 18, 6784–6791 (2012).
Baselga, J. et al. Relationship between tumor biomarkers (BM) and efficacy in EMILIA, a phase III study of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC). Cancer Res. 73, LB-63 (2013).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69, 9330–9336 (2009).
Kang, J. C. et al. Engineering a HER2-specific antibody-drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 37, 523–526 (2019).
Li, B. T. et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 10, 674–687 (2020).
Brevet, M., Arcila, M. & Ladanyi, M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J. Mol. Diagn. 12, 169–176 (2010).
Hamblett, K. J. et al. AMG 595, an anti-EGFRvIII antibody–drug conjugate, induces potent antitumor activity against EGFRvIII-expressing glioblastoma. Mol. Cancer Ther. 14, 1614–1624 (2015).
Comer, F., Gao, C. & Coats, S. in Innovations for Next-Generation Antibody-Drug Conjugates (ed. Damelin, M.) 267–280 (Springer International Publishing, 2018).
Li, J. Y. et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell 35, 948–949 (2019).
de Goeij, B. E. et al. Efficient payload delivery by a bispecific antibody-drug conjugate targeting HER2 and CD63. Mol. Cancer Ther. 15, 2688–2697 (2016).
Andreev, J. et al. Bispecific antibodies and antibody-drug conjugates (ADCs) bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs. Mol. Cancer Ther. 16, 681–693 (2017).
Zhuang, C. et al. Small molecule-drug conjugates: a novel strategy for cancer-targeted treatment. Eur. J. Med. Chem. 163, 883–895 (2019).
Casi, G. & Neri, D. Antibody-drug conjugates and small molecule-drug conjugates: opportunities and challenges for the development of selective anticancer cytotoxic agents. J. Med. Chem. 58, 8751–8761 (2015).
Whalen, K. A. et al. Targeting the somatostatin receptor 2 with the miniaturized drug conjugate, PEN-221: a potent and novel therapeutic for the treatment of small cell lung cancer. Mol. Cancer Ther. 18, 1926–1936 (2019).
Kumthekar, P. et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin. Cancer Res. 26, 2789–2799 (2020).
Gebleux, R., Stringhini, M., Casanova, R., Soltermann, A. & Neri, D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int. J. Cancer 140, 1670–1679 (2017).
Carneiro, B. A. & El-Deiry, W. S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17, 395–417 (2020).
Mohit, E. & Rafati, S. Chemokine-based immunotherapy: delivery systems and combination therapies. Immunotherapy 4, 807–840 (2012).
Cetinbas, N. M. et al. Tumor cell-intrinsic STING pathway is activated in the presence of cues from immune cells and contributes to the anti-tumor activity of tumor cell-targeted STING agonist antibody-drug conjugates [abstract]. J. Immunother. Cancer 8, A373 (2020).
Moyes, K. et al. A systemically administered, conditionally active TLR8 agonist for the treatment of HER2-expressing tumors. Cancer Res. 79, 3271 (2019).
Witzig, T. E. et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 20, 2453–2463 (2002).
Kaminski, M. S. et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N. Engl. J. Med. 352, 441–449 (2005).
Leahy, M. F., Seymour, J. F., Hicks, R. J. & Turner, J. H. Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphoma. J. Clin. Oncol. 24, 4418–4425 (2006).
Gill, M. R., Falzone, N., Du, Y. & Vallis, K. A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 18, e414–e423 (2017).
Dovgan, I., Koniev, O., Kolodych, S. & Wagner, A. Antibody-oligonucleotide conjugates as therapeutic, imaging, and detection agents. Bioconjug Chem. 30, 2483–2501 (2019).
Perez, H. L. et al. Antibody-drug conjugates: current status and future directions. Drug Discov. Today 19, 869–881 (2014).
Dornan, D. & Settleman, J. in Antibody-Drug Conjugates and Immunotoxins: From Pre-Clinical Development to Therapeutic Applications (ed. Phillips, G.L.) 77–90 (Springer, 2013).
Boshuizen, J. et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat. Med. 24, 203–212 (2018).
Yonesaka, K. et al. An HER3-targeting antibody-drug conjugate incorporating a DNA topoisomerase I inhibitor U3-1402 conquers EGFR tyrosine kinase inhibitor-resistant NSCLC. Oncogene 38, 1398–1409 (2019).
Ponte, J. F. et al. Mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, potentiates the activity of standard of care therapeutics in ovarian cancer models. Neoplasia 18, 775–784 (2016).
O’Malley, D. M. et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 157, 379–385 (2020).
Moore, K. N. et al. Phase 1b study of anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 158, 631–639 (2020).
Saatci, O. et al. Targeting PLK1 overcomes T-DM1 resistance via CDK1-dependent phosphorylation and inactivation of Bcl-2/xL in HER2-positive breast cancer. Oncogene 37, 2251–2269 (2018).
Zhong, H. et al. Improved therapeutic window in BRCA-mutant tumors with antibody-linked pyrrolobenzodiazepine dimers with and without PARP inhibition. Mol. Cancer Ther. 18, 89–99 (2019).
Cardillo, T. M. et al. Synthetic lethality exploitation by an anti-trop-2-SN-38 antibody-drug conjugate, IMMU-132, Plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin. Cancer Res. 23, 3405–3415 (2017).
Gerber, H. P., Sapra, P., Loganzo, F. & May, C. Combining antibody-drug conjugates and immune-mediated cancer therapy: What to expect? Biochem. Pharmacol. 102, 1–6 (2016).
Emens, L. A. et al. Results from KATE2, a randomized phase 2 study of atezolizumab (atezo) plus trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+advanced breast cancer (BC). Cancer Res. 79, PD3-01 (2019).
Rosenberg, J. E. et al. Study EV-103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 38, 441–441 (2020).
Macpherson, I. R. & Cassidy, J. Challenges in combinational oncology studies. Pharm. Med. 22, 85–97 (2008).
Acknowledgements
All authors acknowledge support from the NCI Cancer Center Support Grant P30-CA008748. J.Z.D. acknowledges support from the Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746 and a 2020 Conquer Cancer–Breast Cancer Research Foundation Young Investigator Award. S.C. acknowledges support from the Breast Cancer Research Foundation. The authors thank Linda Vahdat and Pedram Razavi of Memorial Sloan Kettering Cancer Center for editorial assistance and appreciate the helpful comments and suggestions provided by the journal editors and reviewers.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to each stage of the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.Z.D. has received Honoraria from OncLive. S.M. has received institutional research support from AstraZeneca, Daiichi Sankyo, Genentech, Novartis and Seattle Genetics; has participated in consulting/advisory boards for AstraZeneca, Daiichi Sankyo, Genentech, Macrogenics and Seattle Genetics; and has received speakers’ bureau from AstraZeneca, Daiichi Sankyo, Genentech and Seattle Genetics. S.C. has received consulting fees from Eli Lilly, Novartis and Paige.ai, and has received research support (via his institution) from Daiichi-Sankyo, Eli Lilly, Novartis and Sanofi.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks Howard Burris III, Yasuhiro Matsumura, Dhaval K. Shah and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Drago, J.Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol 18, 327–344 (2021). https://doi.org/10.1038/s41571-021-00470-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00470-8
This article is cited by
-
A review of the clinical efficacy of FDA-approved antibody‒drug conjugates in human cancers
Molecular Cancer (2024)
-
The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors
Journal of Hematology & Oncology (2024)
-
Drug conjugates for the treatment of lung cancer: from drug discovery to clinical practice
Experimental Hematology & Oncology (2024)
-
Decoding of the surfaceome and endocytome in primary glioblastoma cells identifies potential target antigens in the hypoxic tumor niche
Acta Neuropathologica Communications (2024)
-
Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives
Nature Reviews Clinical Oncology (2024)