Abstract
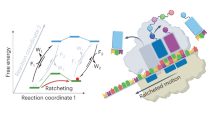
Interrupted reactions reroute established processes to new and often unanticipated end points. Of particular interest are the cases in which a known reactive intermediate takes on a new reaction pathway, either because this pathway is lower in energy or because the conventional pathway is no longer available. Through analysis of documented cases, we aim to dissect the known interrupted reactions and trace their mechanistic origins. As new chemical processes are being discovered at a seemingly ever-increasing pace, it is likely that new interrupted reactions will continue to emerge. Our hope is that the cases considered in this Review will help identify new classes of these fascinating transformations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yudin, A. K. Synthetic half-reactions. Chem. Sci. 11, 12423–12427 (2020).
Nazarov, I. N. & Zaretskaya, I. I. Acetylene derivatives, XVII. Hydration of hydrocarbons of the divinylacetylene series. Izv. Akad. Nauk. SSSR Ser. Khim. 1941, 211–224 (1941).
Vinogradov, M. G., Turova, O. V. & Zlotin, S. G. Nazarov reaction: current trends and recent advances in the synthesis of natural compounds and their analogs. Org. Biomol. Chem. 15, 8245–8269 (2017).
Yadykov, A. V. & Shirinian, V. Z. Recent advances in the interrupted Nazarov reaction. Adv. Synth. Catal. 362, 702–723 (2020).
Vorländer, D. & Schroedter, M. Einwirkung von schwefelsäure und essigsäureanhydrid auf dibenzalaceton. Ber. Dtsch. Chem. Ges. 36, 1490–1497 (1903).
Bender, J. A., Blize, A. E., Browder, C. C., Giese, S. & West, F. G. Highly diastereoselective cycloisomerization of acyclic trienones. The interrupted Nazarov reaction. J. Org. Chem. 63, 2430–2431 (1998).
Giese, S., Kastrup, L., Stiens, D. & West, F. G. Intermolecular trapping of the Nazarov intermediate: domino electrocyclization/[3+2] cycloadditions with allylsilanes. Angew. Chem. Int. Ed. 39, 1970–1973 (2000).
Wang, Y., Schill, B. D., Arif, A. M. & West, F. G. Formal intermolecular 4 + 4 approach to cyclooctanoids: 4 + 3 capture of the Nazarov oxyallyl intermediate with simple 1,3-dienes. Org. Lett. 5, 2747–2750 (2003).
Harmata, M., Huang, C., Roosehenas, P. & Schreiner, P. R. An interrupted [4+3] cycloaddition reaction: a hydride shift (ene reaction) intervenes. Angew. Chem. Int. Ed. 47, 8696–8699 (2008).
Browder, C. C., Marmsäter, F. P. & West, F. G. Highly efficient trapping of the Nazarov intermediate with substituted arenes. Org. Lett. 3, 3033–3035 (2001).
Rieder, C. J., Fradette, R. J. & West, F. G. Heteroaromatic trapping of tricyclic 2-oxidocyclopentenyl cations: a surprisingly efficient example of intermolecular interrupted Nazarov reaction. Heterocycles 80, 1413–1427 (2009).
Rieder, C. J., Fradette, R. J. & West, F. G. Construction of aryl-substituted triquinanes through the interrupted Nazarov reaction. Chem. Commun.2008, 1572–1574 (2008).
Shirinian, V. Z. et al. Triaryl-substituted divinyl ketones cyclization: Nazarov reaction versus Friedel–Crafts electrophilic substitution. Org. Lett. 18, 6260–6263 (2016).
Kwon, Y., Schatz, D. J. & West, F. G. 1,4-Diketones from cross-conjugated dienones: potassium permanganate-interrupted Nazarov reaction. Angew. Chem. Int. Ed. 54, 9940–9943 (2015).
Dhoro, F. & Tius, M. A. Interrupted Nazarov cyclization on silica gel. J. Am. Chem. Soc. 127, 12472–12473 (2005).
Dhoro, F., Kristensen, T. E., Stockmann, V., Yap, G. P. A. & Tius, M. A. Asymmetric amine-intercepted Nazarov cyclization. J. Am. Chem. Soc. 129, 7256–7257 (2007).
Zhang, W. et al. Intercepted retro-Nazarov reaction: syntheses of amidino-rocaglate derivatives and their biological evaluation as eIF4A inhibitors. J. Am. Chem. Soc. 141, 12891–12900 (2019).
Song, D., Rostami, A. & West, F. G. Domino electrocyclization/azide-capture/Schmidt rearrangement of dienones: one-step synthesis of dihydropyridones from simple building blocks. J. Am. Chem. Soc. 129, 12019–12022 (2007).
Rostami, A., Wang, Y., Arif, A. M., McDonald, R. & West, F. G. Intramolecular azide trapping of the Nazarov intermediate: formation of peroxy-bridged indolizidinones via a deep-seated rearrangement and aerobic oxidation. Org. Lett. 9, 703–706 (2007).
Schatz, D. J., Kwon, Y., Scully, T. W. & West, F. G. Interrupting the Nazarov cyclization with bromine. J. Org. Chem. 81, 12494–12498 (2016).
Marx, V. M. & Burnell, D. J. Nazarov cyclizations of an allenyl vinyl ketone with interception of the oxyallyl cation intermediate for the formation of carbon–carbon bonds. J. Am. Chem. Soc. 132, 1685–1689 (2010).
Riveira, M. J., Marsili, L. A. & Mischne, M. P. The iso-Nazarov reaction. Org. Biomol. Chem. 15, 9255–9274 (2017).
Marques, A.-S., Coeffard, V., Chataigner, I., Vincent, G. & Moreau, X. Iron-mediated domino interrupted iso-Nazarov/dearomative (3 + 2)-cycloaddition of electrophilic indoles. Org. Lett. 18, 5296–5299 (2016).
William, R., Wang, S., Ding, F., Arviana, E. S. & Liu, X.-W. Interrupted imino-Nazarov cyclization of 1-aminopentadienyl cation and related cascade process. Angew. Chem. Int. Ed. 53, 10742–10746 (2014).
Marques, A.-S. et al. A fused hexacyclic ring system: diastereoselective polycyclization of 2,4-dienals through an interrupted iso-Nazarov reaction. Angew. Chem. Int. Ed. 58, 9969–9973 (2019).
Fuson, R. C. The principle of vinylogy. Chem. Rev. 16, 1–27 (1935).
Miller, A. K. & Trauner, D. Total synthesis of (±)-photodeoxytridachione through a Lewis acid catalyzed cyclization. Angew. Chem. Int. Ed. 42, 549–552 (2003).
Riveira, M. J. & Mischne, M. P. An interrupted vinylogous iso-Nazarov reaction: cycloisomerization of conjugated trienones to cyclopenta[b]furan derivatives. J. Org. Chem. 79, 8244–8254 (2014).
Ugi, I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. 1, 8–21 (1962).
Dömling, A. & Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39, 3168–3210 (2000).
Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 42, 463–472 (2009).
El Kaïm, L. & Grimaud, L. Beyond the Ugi reaction: less conventional interactions between isocyanides and iminium species. Tetrahedron 65, 2153–2171 (2009).
El Kaïm, L., Grimaud, L. & Oble, J. Phenol Ugi–Smiles systems: strategies for the multicomponent N-arylation of primary amines with isocyanides, aldehydes, and phenols. Angew. Chem. Int. Ed. 44, 7961–7964 (2005).
Giustiniano, M. et al. Interrupted Ugi and Passerini reactions: an underexplored treasure island. Synthesis 50, 3549–3570 (2018).
Deyrup, J. A., Vestling, M. M., Hagan, W. V. & Yun, H. Y. Reactions of imines with t-butyl isocyanide. Tetrahedron 25, 1467–1478 (1969).
Schneekloth, J. S., Kim, J. Jr. & Sorensen, E. J. An interrupted Ugi reaction enables the preparation of substituted indoxyls and aminoindoles. Tetrahedron 65, 3096–3101 (2009).
Tron, G. C. Off the beaten track: the use of secondary amines in the Ugi reaction. Eur. J. Org. Chem. 2013, 1849–1859 (2013).
Giovenzana, G. B., Tron, G. C., Di Paola, S., Menegotto, I. G. & Pirali, T. A mimicry of primary amines by bis-secondary diamines as components in the Ugi four-component reaction. Angew. Chem. Int. Ed. 45, 1099–1102 (2006).
Ramazani, A. & Rezaei, A. Novel one-pot, four-component condensation reaction: an efficient approach for the synthesis of 2,5-disubstituted 1,3,4-oxadiazole derivatives by a Ugi-4CR/aza-Wittig sequence. Org. Lett. 12, 2852–2855 (2010).
Hili, R., Rai, V. & Yudin, A. K. Macrocyclization of linear peptides enabled by amphoteric molecules. J. Am. Chem. Soc. 132, 2889–2891 (2010).
Boltjes, A. & Dömling, A. The Groebke-Blackburn-Bienaymé reaction. Eur. J. Org. Chem. 2019, 7007–7049 (2019).
Groebke, K., Weber, L. & Mehlin, F. Synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines by a novel three-component condensation. Synlett 1998, 661–663 (1998).
Salunke, D. B. et al. Structure–activity relationships in human toll-like receptor 8-active 2,3-diamino-furo[2,3-c]pyridines. J. Med. Chem. 55, 8137–8151 (2012).
Wrobleski, A., Coombs, T. C., Huh, C. W., Li, S.-W. & Aubé, J. The Schmidt reaction. Org. React. 78, 1–320 (2012).
Schmidt, K. F. Über den imin-rest. Ber. Dtsch. Chem. Ges. 57, 704–706 (1924).
Motiwala, H. F., Charaschanya, M., Day, V. W. & Aubé, J. Remodeling and enhancing Schmidt reaction pathways in hexafluoroisopropanol. J. Org. Chem. 81, 1593–1609 (2016).
Charaschanya, M., Li, K., Motiwala, H. F. & Aubé, J. An interrupted Schmidt reaction: C–C bond formation arising from nitrilium ion capture. Org. Lett. 20, 6354–6358 (2018).
Crozet, D., Urrutigoïty, M. & Kalck, P. Recent advances in amine synthesis by catalytic hydroaminomethylation of alkenes. ChemCatChem 3, 1102–1118 (2011).
Chen, C. et al. Rhodium/Yanphos-catalyzed asymmetric interrupted intramolecular hydroaminomethylation of trans-1,2-disubstituted alkenes. J. Am. Chem. Soc. 138, 9017–9020 (2016).
Pummerer, R. Über phenyl-sulfoxyessigsäure. Ber. Dtsch. Chem. Ges. 42, 2282–2291 (1909).
Padwa, A. & Kuethe, J. T. Additive and vinylogous Pummerer reactions of amido sulfoxides and their use in the preparation of nitrogen containing heterocycles. J. Org. Chem. 63, 4256–4268 (1998).
Yorimitsu, H. Cascades of interrupted Pummerer reaction-sigmatropic rearrangement. Chem. Rec. 17, 1156–1167 (2017).
Omura, K. & Swern, D. Oxidation of alcohols by “activated” dimethyl sulfoxide. a preparative, steric and mechanistic study. Tetrahedron 34, 1651–1660 (1978).
Corey, E. J. & Kim, C. U. New and highly effective method for the oxidation of primary and secondary alcohols to carbonyl compounds. J. Am. Chem. Soc. 94, 7586–7587 (1972).
Parikh, J. & Doering, W. V. E. Sulfur trioxide in the oxidation of alcohols by dimethyl sulfoxide. J. Am. Chem. Soc. 89, 5505–5507 (1967).
Tayu, M., Higuchi, K., Inaba, M. & Kawasaki, T. Sulfoxide-TFAA and nucleophile combination as new reagent for aliphatic C–H functionalization at indole 2α-position. Org. Biomol. Chem. 11, 496–502 (2013).
Nenajdenko, V. G., Vertelezkij, P. V., Gridnev, I. D., Shevchenko, N. E. & Balenkova, E. S. Reaction of dimethyl sulfide ditriflate with alkenes. Synthesis of sulfur derivatives of nortricyclane. Tetrahedron 53, 8173–8180 (1997).
Luo, H., Hu, G. & Li, P. Sulfur-mediated allylic C–H arylation, epoxidation, and aziridination. J. Org. Chem. 84, 10569–10578 (2019).
Aukland, M. H. et al. An interrupted Pummerer/nickel-catalysed cross-coupling sequence. Angew. Chem. Int. Ed. 57, 9785–9789 (2018).
Yoshida, S., Yorimitsu, H. & Oshima, K. 2-(2,2,2-Trifluoroethylidene)-1,3-dithiane monoxide as a trifluoromethylketene equivalent. Org. Lett. 11, 2185–2188 (2009).
Huang, X., Klimczyk, S. & Maulide, N. Charge-accelerated sulfonium [3,3]-sigmatropic rearrangements. Synthesis 44, 175–183 (2012).
Huang, X. & Maulide, N. Sulfoxide-mediated α-arylation of carbonyl compounds. J. Am. Chem. Soc. 133, 8510–8513 (2011).
Eberhart, A. J., Imbriglio, J. E. & Procter, D. J. Nucleophilic ortho allylation of aryl and heteroaryl sulfoxides. Org. Lett. 13, 5882–5885 (2011).
Eberhart, A. J. & Procter, D. J. Nucleophilic ortho-propargylation of aryl sulfoxides: an interrupted Pummerer/allenyl thio-Claisen rearrangement sequence. Angew. Chem. Int. Ed. 52, 4008–4011 (2013).
Fernández-Salas, J. A., Eberhart, A. J. & Procter, D. J. Metal-free CH–CH-type cross-coupling of arenes and alkynes directed by a multifunctional sulfoxide group. J. Am. Chem. Soc. 138, 790–793 (2016).
Yanagi, T. et al. Metal-free approach to biaryls from phenols and aryl sulfoxides by temporarily sulfur-tethered regioselective C–H/C–H coupling. J. Am. Chem. Soc. 138, 14582–14585 (2016).
Shang, L. et al. Redox-neutral α-arylation of alkyl nitriles with aryl sulfoxides: a rapid electrophilic rearrangement. J. Am. Chem. Soc. 139, 4211–4217 (2017).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Shrives, H. J., Fernández-Salas, J. A., Hedtke, C., Pulis, A. P. & Procter, D. J. Regioselective synthesis of C3 alkylated and arylated benzothiophenes. Nat. Commun. 8, 14801 (2017).
He, Z. et al. Synthesis of C2 substituted benzothiophenes via an interrupted Pummerer/[3,3]-sigmatropic/1,2-migration cascade of benzothiophene S-oxides. Angew. Chem. Int. Ed. 57, 5759–5764 (2018).
He, Z., Pulis, A. P. & Procter, D. J. The interrupted Pummerer reaction in a sulfoxide-catalyzed oxidative coupling of 2-naphthols. Angew. Chem. Int. Ed. 58, 7813–7817 (2019).
Fischer, E. & Hess, O. Synthese von indolderivaten. Ber. Dtsch. Chem. Ges. 17, 559–568 (1884).
Robinson, B. The Fischer indole synthesis. Chem. Rev. 63, 373–401 (1963).
Humphrey, G. R. & Kuethe, J. T. Practical methodologies for the synthesis of indoles. Chem. Rev. 106, 2875–2911 (2006).
Grandberg, I. I., Zuyanoa, T. I., Afonina, N. I. & Ivanova, T. A. New method of synthesizing important biogenic amines. Dokl. Akad. Nauk. SSSR 176, 583–585 (1967).
Grandberg, I. I. & Tokmakov, G. Indoles. XLVIL. Synthesis of 2,3,3a,8a-tetrahydrofuro[2,3-b]indole derivatives. Chem. Heterocycl. Compd. 11, 176–179 (1975).
Britten, A. Z., Bardsley, W. G. & Hill, C. M. Furano- and pyrano-indolines-model compounds for indole alkaloid studies. Tetrahedron 27, 5631–5639 (1971).
Rosenmund, P. & Sadri, E. Beiträge zur chemie des indols, XII. Synthesen eserinahnlicher verbindungen, III; neue an N-1 und N-8 substituierte derivate des desoxyeserolins sowie eine neue synthese des eserthols. Liebigs Ann. Chem. 1979, 927–943 (1979).
Boal, B. W., Schammel, A. W. & Garg, N. K. An interrupted Fischer indolization approach toward fused indoline-containing natural products. Org. Lett. 11, 3458–3461 (2009).
Mo, Y., Zhao, J., Chen, W. & Wang, Q. Recent advance of the application of interrupted Fischer indolization toward bioactive indoline alkaloids. Res. Chem. Intermed. 41, 5869–5877 (2015).
Susick, R. B., Morrill, L. A., Picazo, E. & Garg, N. K. Pardon the interruption: a modification of Fischer’s venerable reaction for the synthesis of heterocycles and natural products. Synlett 28, 1–11 (2017).
Varga, S. et al. Total syntheses of (−)-minovincine and (−)-aspidofractinine through a sequence of cascade reactions. Angew. Chem. Int. Ed. 59, 13547–13551 (2020).
Maligres, P. E. et al. Synthesis of the orally active spiroindoline-based growth hormone secretagogue, MK-677. Tetrahedron 53, 10983–10992 (1997).
Moreno, J., Picazo, E., Morrill, L. A., Smith, J. M. & Garg, N. K. Enantioselective total syntheses of akuammiline alkaloids (+)-strictamine, (−)-2(S)-cathafoline, and (−)-aspidophylline A. J. Am. Chem. Soc. 138, 1162–1165 (2016).
De Graaff, C. et al. Asymmetric synthesis of tetracyclic pyrroloindolines and constrained tryptamines by a switchable cascade reaction. Angew. Chem. Int. Ed. 54, 14133–14136 (2015).
Wang, H.-Y. & Anderson, L. L. Interrupted Fischer-indole intermediates via oxyarylation of alkenyl boronic acids. Org. Lett. 15, 3362–3365 (2013).
Pictet, A. & Spengler, T. Über die bildung von isochinolin-derivaten durch einwirkung von methylal auf phenyl-äthylamin, phenyl-alanin und tyrosin. Ber. Dtsch. Chem. Ges. 44, 2030–2036 (1911).
Zheng, C. & You, S.-L. Exploring the chemistry of spiroindolenines by mechanistically-driven reaction development: asymmetric Pictet–Spengler-type reactions and beyond. Acc. Chem. Res. 53, 974–987 (2020).
Williams, J. R. & Unger, L. R. Biogenetically patterned synthesis of the spiro[indoline-3,4′-proline] system. J. Chem. Soc. D 1970, 1605–1606 (1970).
Gabriel, P., Gregory, A. W. & Dixon, D. J. Iridium-catalyzed aza-spirocyclization of indole-tethered amides: an interrupted Pictet–Spengler reaction. Org. Lett. 21, 6658–6662 (2019).
Woodward, R. B. et al. The total synthesis of strychnine. J. Am. Chem. Soc. 76, 4749–4751 (1954).
Harley-Mason, J. & Waterfield, W. R. The synthesis, rearrangement and certain reactions of 1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydro-β-carboline and its 9-methyl analogue. Tetrahedron 19, 65–76 (1963).
Nakagawa, M., Liu, J. J. & Hino, T. Total synthesis of (−)-eudistomin L and (−)-debromoeudistomin L. J. Am. Chem. Soc. 111, 2721–2722 (1989).
Hili, R. & Yudin, A. K. Readily available unprotected amino aldehydes. J. Am. Chem. Soc. 128, 14772–14773 (2006).
Trzupek, J. D., Lee, D., Crowley, B. M., Marathias, V. M. & Danishefsky, S. J. Total synthesis of enantiopure phalarine via a stereospecific Pictet–Spengler reaction: traceless transfer of chirality from l-tryptophan. J. Am. Chem. Soc. 132, 8506–8512 (2010).
Liu, F. & Movassaghi, M. Electrophilic carbonyl activation: competing condensative cyclizations of tryptamine derivatives. Tetrahedron Lett. 56, 2995–3000 (2015).
Bischler, A. & Napieralski, B. Zur kenntniss einer neuen isochinolinsynthese. Ber. Dtsch. Chem. Ges. 26, 1903–1908 (1893).
Medley, J. W. & Movassaghi, M. Synthesis of spirocyclic indolines by interruption of the Bischler–Napieralski reaction. Org. Lett. 15, 3614–3617 (2013).
Povarov, L. S. & Mikhailov, B. M. A new type of diene condensation reaction. Izv. Akad. Nauk. SSR Ser. Khim. 1963, 955–956 (1963).
Bello Forero, J. S., Jones, J. Jr. & da Silva, F. M. The Povarov reaction as a versatile strategy for the preparation of 1,2,3,4-tetrahydroquinoline derivatives: an overview. Curr. Org. Synth. 13, 157–175 (2016).
Domingo, L. R., Aurell, M. J., Sáez, J. A. & Mekelleche, S. M. Understanding the mechanism of the Povarov reaction. A DFT study. RSC Adv. 4, 25268–25278 (2014).
Fochi, M., Caruana, L. & Bernardi, L. Catalytic asymmetric aza-Diels–Alder reactions: the Povarov cycloaddition reaction. Synthesis 46, 135–157 (2014).
Yadav, J. S., Reddy, B. V. S., Sekhar, K. C. & Geetha, V. Sc(OTf)3-catalyzed diastereoselective synthesis of 3,4-dihydro-4-amino-2H-1-benzopyrans. Tetrahedron Lett. 42, 4405–4407 (2001).
Dong, S., Liu, X., Zhang, Y., Lin, L. & Feng, X. Asymmetric synthesis of 3,4-diaminochroman-2-ones promoted by guanidine and bisguanidium salt. Org. Lett. 13, 5060–5063 (2011).
Isambert, N., Cuz, M., Arévalo, M. J., Gómez, E. & Lavilla, R. Enol esters: versatile substrates for Mannich-type multicomponent reactions. Org. Lett. 9, 4199–4202 (2007).
Cala, L., Mendoza, A., Fañanás, F. J. & Rodríguez, F. A catalytic multicomponent coupling reaction for the enantioselective synthesis of spiroacetals. Chem. Commun. 49, 2715–2717 (2013).
Kobayashi, S., Ishitani, H. & Nagayama, S. Ln(OTf)3- or Sc(OTf)3-catalyzed three components coupling reactions between aldehydes, amines, and dienes or alkenes. Efficient syntheses of pyridine and quinoline derivatives. Chem. Lett. 24, 423–424 (1995).
Preciado, S., Vicente-García, E., Llabrés, S., Luque, F. J. & Lavilla, R. Exploration of forbidden Povarov processes as a source of unexpected reactivity: a multicomponent Mannich–Ritter transformation. Angew. Chem. Int. Ed. 51, 6874–6877 (2012).
Meyer, K. H. & Schuster, K. Umlagerung tertiärer äthinyl-carbinole in ungesättigte ketone. Ber. Dtsch. Chem. Ges. 55, 819–823 (1922).
Liu, Z. et al. InCl3-catalyzed propargylation of indoles and phenols with propargylic acetates: application to the syntheses of benzofurans and naphthofurans. Synthesis 13, 1961–1969 (2007).
Zhao, W. & Carreira, E. M. Facile one-pot synthesis of photochromic pyrans. Org. Lett. 5, 4153–4154 (2003).
Kaiser, D., Veiros, L. F. & Maulide, N. Redox-neutral arylations of vinyl cation intermediates. Adv. Synth. Catal. 359, 64–77 (2017).
Tharra, P. & Baire, B. The Z-enoate assisted, Meyer–Schuster rearrangement cascade: unconventional synthesis of α-arylenone esters. Chem. Commun. 52, 12147–12150 (2016).
Roy, D., Tharra, P. & Baire, B. Intercepted Meyer–Schuster rearrangements in organic synthesis. Asian J. Org. Chem. 7, 1015–1032 (2018).
Belgacem, J., Kress, J. & Osborn, J. A. On the allylic rearrangements in metal oxo complexes: mechanistic and catalytic studies on MoO2 (allyloxo)2(CH3CN)2 and analogous complexes. J. Am. Chem. Soc. 114, 1501–1502 (1992).
Trost, B. M. & Oi, S. Atom economy: aldol-type products by vanadium-catalyzed additions of propargyl alcohols and aldehydes. J. Am. Chem. Soc. 123, 1230–1231 (2001).
Trost, B. M. & Chung, C. K. Vanadium-catalyzed addition of propargyl alcohols and imines. J. Am. Chem. Soc. 128, 10358–10359 (2006).
Baeyer, A. & Villiger, V. Einwirkung des caro’schen reagens auf ketone. Ber. Dtsch. Chem. Ges. 32, 3625–3633 (1899).
Ten Brink, G.-J., Arends, I. W. C. E. & Sheldon, R. A. The Baeyer–Villiger reaction: new developments toward greener procedures. Chem. Rev. 104, 4105–4124 (2004).
Yu, X. et al. Oxidative rearrangement of malondialdehyde: substrate scope and mechanistic insights. RSC Adv. 4, 53397–53401 (2014).
Vil’, V. A. et al. Interrupted Baeyer–Villiger rearrangement: building a stereoelectronic trap for the Criegee intermediate. Angew. Chem. Int. Ed. 57, 3372–3376 (2018).
Erhardt, S. et al. Model studies of β-scission ring-opening reactions of cyclohexyloxy radicals: application to thermal rearrangements of dispiro-1,2,4-trioxanes. Org. Lett. 9, 5569–5572 (2007).
Ravi, M. et al. Synthesis of 1,2,4-trioxepanes and 1,2,4-trioxanes via H2O2-mediated reaction of tertiary carbinols. Synlett 24, 173–176 (2013).
Bloodworth, A. J. & Shah, A. Synthesis of 1,2,4-trioxanes via intramolecular oxymercuriation. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39910000947 (1991).
Booldworth, A. J. & Tallant, N. A. 1,2,4-Trioxane versus 1,2-dioxolane formation in the mercury(II) acetate-mediated cyclisation of hemiperoxyacetals derived from allylic hydroperoxides. J. Chem. Soc. Chem. Commun. 1992, 428–429 (1992).
Schweitzer-Chaput, B., Demaerel, J., Engler, H. & Klussmann, M. Acid-catalyzed oxidative radical addition of ketones to olefins. Angew. Chem. Int. Ed. 53, 8737–8740 (2014).
Schweitzer-Chaput, B., Kurtén, T. & Klussmann, M. Acid-mediated formation of radicals or Baeyer–Villiger oxidation from Criegee adducts. Angew. Chem. Int. Ed. 54, 11848–11851 (2015).
Schweitzer-Chaput, B., Boess, E. & Klussmann, M. Acid-catalyzed activation of peroxyketals: tunable radical initiation at ambient temperature and below. Org. Lett. 18, 4944–4947 (2016).
Wang, D.-S., Chen, Q.-A., Lu, S.-M. & Zhou, Y.-G. Asymmetric hydrogenation of heteroarenes and arenes. Chem. Rev. 112, 2557–2590 (2012).
Glorius, F., Spielkamp, N., Holle, S., Goddard, R. & Lehmann, C. W. Efficient asymmetric hydrogenation of pyridines. Angew. Chem. Int. Ed. 43, 2850–2852 (2004).
Grozavu, A. et al. The reductive C3 functionalization of pyridinium and quinolinium salts through iridium-catalysed interrupted transfer hydrogenation. Nat. Chem. 11, 242–247 (2019).
Hepburn, H. B. & Donohoe, T. J. Reductive hydroxymethylation of 4-heteroarylpyridines. Chem. Eur. J. 26, 1963–1967 (2020).
Marinic, B., Hepburn, H. B., Grozavu, A., Dow, M. & Donohoe, T. J. Single point activation of pyridines enables reductive hydroxymethylation. Chem. Sci. 12, 742–746 (2021).
Shen, D. et al. Hydrogen-borrowing and interrupted-hydrogen-borrowing reactions of ketones and methanol catalyzed by iridium. Angew. Chem. Int. Ed. 54, 1642–1645 (2015).
Grozavu, A., Hepburn, H. B., Bailey, E. P., Lindsay-Scott, P. J. & Donohoe, T. J. Rhodium catalysed C-3/5 methylation of pyridines using temporary dearomatisation. Chem. Sci. 11, 8595–8599 (2020).
Wagener, T., Lückemeier, L., Daniliuc, C. G. & Glorius, F. Interrupted pyridine hydrogenation: asymmetric synthesis of δ-lactams. Angew. Chem. Int. Ed. 60, 6425–6429 (2021).
Ravindar, L. et al. Carbonyl-olefin metathesis: a key review. Org. Chem. Front. 5, 1381–1391 (2018).
Ludwig, J. R. et al. Interrupted carbonyl-olefin metathesis via oxygen atom transfer. Science 136, 1363–1369 (2018).
McFarlin, A. T., Watson, R. B., Zehnder, T. E. & Schindler, C. S. Interrupted carbonyl-alkyne metathesis. Adv. Synth. Catal. 362, 365–369 (2020).
Wei, Y. & Shi, M. Recent advances in organocatalytic asymmetric Morita–Baylis–Hillman/aza-Morita–Baylis–Hillman reactions. Chem. Rev. 113, 6659–6690 (2013).
Gu, J. et al. Interrupted Morita–Baylis–Hillman-type reaction of α-substituted activated olefins. Org. Lett. 20, 2088–2091 (2018).
Aroyan, C. E., Dermenci, A. & Miller, S. J. The Rauhut–Currier reaction: a history and its synthetic application. Tetrahedron 65, 4069–4084 (2009).
McDougal, N. T. & Schaus, S. E. Highly diastereoselective synthesis of bicyclo[3.2.1]octenones through phosphine-mediated condensations of 1,4-dien-3-ones. Angew. Chem. Int. Ed. 45, 3117–3119 (2006).
Tambar, U. K. & Stoltz, B. M. The direct acyl-alkylation of arynes. J. Am. Chem. Soc. 127, 5340–5341 (2005).
Mohanan, K., Coquerel, Y. & Rodriguez, J. Transition-metal-free α-arylation of β-keto amides via an interrupted insertion reaction of arynes. Org. Lett. 14, 4686–4689 (2012).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Popescu, M. V., Mekereeya, A., Alegre-Requena, J. V., Paton, R. S. & Smith, M. D. Visible-light-mediated heterocycle functionalization via geometrically interrupted [2+2] cycloaddition. Angew. Chem. Int. Ed. 59, 23020–23024 (2020).
Mori, K., Sueoka, S. & Akiyama, T. Expeditious construction of a carbobicyclic skeleton via sp3-C–H functionalization: hydride shift from an aliphatic tertiary position in an internal redox process. J. Am. Chem. Soc. 133, 2424–2426 (2011).
Mentel, M. & Breinbauer, R. The Witkop-Winterfeldt-oxidation of indoles. Curr. Org. Chem. 11, 159–176 (2007).
Lu, X. et al. Assembly of C3a-peroxylated pyrroloindolines via interrupted Witkop oxidation. Org. Lett. 20, 7937–7941 (2018).
Mills, A. D., Nazer, M. Z., Haddadin, M. J. & Kurth, M. J. N,N-bond-forming heterocyclization: synthesis of 3-alkoxy-2H-indazoles. J. Org. Chem. 71, 2687–2689 (2006).
Zhu, J. S. et al. Diverting reactive intermediates toward unusual chemistry: unexpected anthranil products from Davis–Beirut reaction. J. Org. Chem. 82, 10875–10882 (2017).
Dimroth, O. Ueber eine synthese von derivaten des 1.2.3-triazols. Ber. Dtsch. Chem. Ges. 35, 1029–1038 (1902).
El Ashry, E. S. H., Nadeem, S., Shah, M. R. & El Kilany, Y. Recent advances in the Dimroth rearrangement: a valuable tool for the synthesis of heterocycles. Adv. Heterocycl. Chem. 101, 161–228 (2010).
Zhou, Z.-W. et al. A concise construction of 12H-benzo[4,5]thiazolo[2,3-b]quinazolin-12-ones via an unusual TBHP/Na2CO3 promoted cascade oxidative cyclization and interrupted Dimroth rearrangement. Chem. Commun. 53, 1056–1059 (2017).
Majetich, G. & Wheless, K. Remote intramolecular free radical functionalizations: an update. Tetrahedron 51, 7095–7129 (1995).
Short, M. A., Blackburn, J. M. & Roizen, J. L. Sulfamate esters guide selective radical-mediated chlorination of aliphatic C–H bonds. Angew. Chem. Int. Ed. 57, 296–299 (2018).
Sathyamoorthi, S., Banerjee, S., Du Bois, J., Burns, N. Z. & Zare, R. N. Site-selective bromination of sp3 C–H bonds. Chem. Sci. 9, 100–104 (2018).
Duhamel, T., Martínez, M. D., Sideri, I. K. & Muñiz, K. 1,3-Diamine formation from an interrupted Hofmann–Löffler reaction: iodine catalyst turnover through Ritter-type amination. ACS Catal. 9, 7741–7745 (2019).
Kumar, G., Pradhan, S. & Chatterjee, I. N-centered radical directed remote C–H bond functionalization via hydrogen atom transfer. Chem. Asian J. 15, 651–672 (2020).
Nakamura, I., Yamagishi, U., Song, D., Konta, S. & Yamamoto, Y. Gold- and indium-catalyzed synthesis of 3- and 6-sulfonylindoles from ortho-alkynyl-N-sulfonylanilines. Angew. Chem. Int. Ed. 46, 2284–2287 (2007).
Teo, W. T., Rao, W., Koh, M. J. & Chang, P. W. H. Gold-catalyzed domino aminocyclization/1,3-sulfonyl migration of N-substituted N-sulfonyl-aminobut-3-yn-2-ols to 1-substituted 3-sulfonyl-1H-pyrroles. J. Org. Chem. 78, 7508–7517 (2013).
Wu, F., Chen, L., Wang, Y. & Zhu, S. Gold-catalyzed generation of azafulvenium from an enyne sulfonamide: rapid access to fully substituted pyrroles. Org. Chem. Front. 6, 480–485 (2019).
Astoin, J., Demerseman, P., Riveron, A. & Royer, R. Recherches sur le benzofuranne. LX. Orientation de la dégradation alcaline des aroyl-3 éthyl-2 benzofurannes, selon la nature de leur groupe aroyle. J. Heterocycl. Chem. 14, 867–869 (1977).
Srinivas, K., Sharma, R. & Ramana, C. V. Interrupting base-mediated benzofuran ring transformation with Michael acceptors. J. Org. Chem. 82, 9816–9823 (2017).
Kondrat’eva, G. Y. Diene condensation of oxazole homologs with maliec acid and its anhydride. Khim. Nauka Prom. 2, 666 (1957).
Wenkert, D. et al. N-methylated 5-alkenyloxazolium salt transformations. Org. Lett. 3, 2301–2303 (2001).
Qadoumi, Z., Senior, J., Bergeron-Brlek, M. & Britton, R. Direct access to iminosugars through an interrupted Kondrat’eva reaction. Synlett 24, 2427–2430 (2013).
Acknowledgements
The Natural Sciences and Engineering Research Council of Canada (NSERC) is acknowledged for its financial support. C.-H.T. would like to thank the Connaught Fund (University of Toronto) for funding.
Author information
Authors and Affiliations
Contributions
V.T., C.-H.T. and A.T. researched data and designed the figures. All authors contributed to the writing and editing of this Review.
Corresponding author
Additional information
Dedicated to Professor K. Barry Sharpless on the occasion of his 80th birthday.
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Chemistry thanks F. West and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trudel, V., Tien, CH., Trofimova, A. et al. Interrupted reactions in chemical synthesis. Nat Rev Chem 5, 604–623 (2021). https://doi.org/10.1038/s41570-021-00304-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00304-2