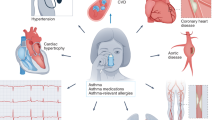
Abstract
Eosinophils are essential innate immune cells in allergic responses. Accumulating evidence indicates that eosinophils also participate in the pathogenesis of cardiovascular diseases (CVDs). In clinical studies, high blood eosinophil counts and eosinophil cationic protein levels have been associated with an increased risk of CVD, including myocardial infarction (MI), cardiac hypertrophy, atrial fibrillation, abdominal aortic aneurysm (AAA) and atherosclerosis. However, low blood eosinophil counts have also been reported to be a risk factor for MI, heart failure, aortic dissection, AAA, deep vein thrombosis, pulmonary embolism and ischaemic stroke. Although these conflicting clinical observations remain unexplained, CVD status, timing of eosinophil data collection, and tissue eosinophil phenotypic and functional heterogeneities might account for these discrepancies. Preclinical studies suggest that eosinophils have protective actions in MI, cardiac hypertrophy, heart failure and AAA. By contrast, cationic proteins and platelet-activating factor from eosinophils have been shown to promote vascular smooth muscle cell proliferation, vascular calcification, thrombomodulin inactivation and platelet activation and aggregation, thereby exacerbating atherosclerosis, atrial fibrillation, thrombosis and associated complications. Therefore, eosinophils seem to promote calcification and thrombosis in chronic CVD but are protective in acute cardiovascular settings. In this Review, we summarize the available clinical and preclinical data on the different roles of eosinophils in CVD.
Key points
-
High blood eosinophil counts and eosinophil cationic protein levels have been suggested to predict a higher risk of cardiovascular disease in humans; however, other studies suggest that low blood eosinophil counts are also predictive of increased risk of cardiovascular disease.
-
Disease status and timing of collection of blood eosinophil data can influence risk assessment, with low blood eosinophil counts being a risk factor for acute cardiovascular events or being detected from early data collection after the events.
-
Low eosinophil counts and high cationic protein levels are associated with a higher risk of thrombosis, ischaemic stroke, deep vein thrombosis and pulmonary embolism.
-
In preclinical models of myocardial infarction, cardiac hypertrophy or heart failure, eosinophil cationic proteins, IL-4 and IL-13 limit cardiomyocyte death and hypertrophy, inhibit cardiac fibrosis and promote M2-like macrophage polarization.
-
Eosinophils might protect against aortic dissection and abdominal aortic aneurysms by controlling angiogenesis, monocyte and macrophage polarization, and aortic vascular and immune cell activation.
-
Eosinophils promote atherogenesis through the production of cationic proteins and platelet-activating factor to stimulate platelets and thrombosis, recruit platelets to the endothelium and induce vascular calcification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Khoury, P., Grayson, P. C. & Klion, A. D. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat. Rev. Rheumatol. 10, 474–483 (2014).
Wen, T. & Rothenberg, M. E. The regulatory function of eosinophils. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.MCHD-0020-2015 (2016).
Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 87, 463–485 (2011).
Takatsu, K. & Nakajima, H. IL-5 and eosinophilia. Curr. Opin. Immunol. 20, 288–294 (2008).
Kouro, T. & Takatsu, K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int. Immunol. 21, 1303–1309 (2009).
Ramirez, G. A. et al. Eosinophils from physiology to disease: a comprehensive review. Biomed. Res. Int. 2018, 9095275 (2018).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Lee, E. H. et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci. Rep. 8, 9894 (2018).
Davoine, F. & Lacy, P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front. Immunol. 5, 570 (2014).
Hams, E., Locksley, R. M., McKenzie, A. N. & Fallon, P. G. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 191, 5349–5353 (2013).
Mesnil, C. et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Invest. 126, 3279–3295 (2016).
Bousquet, J. et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 323, 1033–1039 (1990).
McBrien, C. N. & Menzies-Gow, A. The biology of eosinophils and their role in asthma. Front. Med. 4, 93 (2017).
Hartl, S. et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur. Respir. J. 55, 1901874 (2020).
Liu, C. L. et al. Allergic lung inflammation aggravates angiotensin II-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 36, 69–77 (2016).
Liu, C. L. et al. Allergic lung inflammation promotes atherosclerosis in apolipoprotein E-deficient mice. Transl. Res. 171, 1–16 (2016).
Liu, C. L. et al. Asthma associates with human abdominal aortic aneurysm and rupture. Arterioscler. Thromb. Vasc. Biol. 36, 570–578 (2016).
Guo, J. et al. Allergic asthma is a risk factor for human cardiovascular diseases. Nat. Cardiovasc. Res. 1, 417–430 (2022).
Liu, C. L. et al. Eosinophils protect mice from angiotensin-II perfusion-induced abdominal aortic aneurysm. Circ. Res. 128, 188–202 (2021).
Meng, Z. et al. Cationic proteins from eosinophils bind bone morphogenetic protein receptors promoting vascular calcification and atherogenesis. Eur. Heart J. 44, 2763–2783 (2023).
Welsh, C. et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler. Thromb. Vasc. Biol. 38, 1415–1423 (2018).
Groot, H. E., van Blokland, I. V., Lipsic, E., Karper, J. C. & van der Harst, P. Leukocyte profiles across the cardiovascular disease continuum: a population-based cohort study. J. Mol. Cell. Cardiol. 138, 158–164 (2020).
Nadimi, A. E., Ahmadi, J. & Mehrabian, M. Peripheral eosinophil count and allergy in patients with coronary artery disease. Acta Med. Indones. 40, 74–77 (2008).
Pongdee, T. et al. Rethinking blood eosinophil counts: epidemiology, associated chronic diseases, and increased risks of cardiovascular disease. J. Allergy Clin. Immunol. Glob. 1, 233–240 (2022).
Niccoli, G. et al. Pre-intervention eosinophil cationic protein serum levels predict clinical outcomes following implantation of drug-eluting stents. Eur. Heart J. 30, 1340–1347 (2009).
Quinta, J. B. et al. Cardiovascular adverse effects of anti-IL-5/IL-5Ralpha therapies: a real-world study. J. Allergy Clin. Immunol. Pract. 9, 1411–1413 (2021).
Verdoia, M. et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J. Thromb. Thrombolysis 39, 459–466 (2015).
Shah, A. D., Denaxas, S., Nicholas, O., Hingorani, A. D. & Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart 3, e000477 (2016).
Gao, S. et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 286, 128–134 (2019).
Liu, T. et al. Group 2 innate lymphoid cells protect mouse heart from myocardial infarction injury via interleukin 5, eosinophils, and dendritic cells. Cardiovasc. Res. 119, 1046–1061 (2023).
Zhang, Y. et al. Group 2 innate lymphoid cells protect mice from abdominal aortic aneurysm formation via IL5 and eosinophils. Adv. Sci. 10, e2206958 (2023).
Yang, C. et al. Eosinophils protect pressure overload- and beta-adrenoreceptor agonist-induced cardiac hypertrophy. Cardiovasc. Res. 119, 195–212 (2023).
Liu, J. et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 11, 6396 (2020).
Xu, J. Y. et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc. Res. 118, 2165–2178 (2022).
Yu, X. et al. Innate lymphoid cells promote recovery of ventricular function after myocardial infarction. J. Am. Coll. Cardiol. 78, 1127–1142 (2021).
Knutsson, A. et al. Associations of interleukin-5 with plaque development and cardiovascular events. JACC Basic Transl. Sci. 4, 891–902 (2019).
Gu, L. et al. The relationship between interleukin-4 levels and cardiovascular events in patients with chronic kidney disease. Risk Manag. Healthc. Policy 13, 2371–2377 (2020).
Silveira, A. et al. Plasma IL-5 concentration and subclinical carotid atherosclerosis. Atherosclerosis 239, 125–130 (2015).
Venge, P. et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin. Exp. Allergy 29, 1172–1186 (1999).
Lehrer, R. I. et al. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J. Immunol. 142, 4428–4434 (1989).
Chihara, J. et al. Possible release of eosinophil granule proteins in response to signaling from intercellular adhesion molecule-1 and its ligands. Int. Arch. Allergy Immunol. 108, 52–54 (1995).
Hernnas, J. et al. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur. J. Cell Biol. 59, 352–363 (1992).
Pickett, J. R., Wu, Y., Zacchi, L. F. & Ta, H. T. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc. Res. 119, 2278–2293 (2023).
Barallobre-Barreiro, J. et al. Extracellular matrix in heart failure: role of ADAMTS5 in proteoglycan remodeling. Circulation 144, 2021–2034 (2021).
Halim, T. Y. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Kamijo, S. et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J. Immunol. 190, 4489–4499 (2013).
Doherty, T. A. At the bench: understanding group 2 innate lymphoid cells in disease. J. Leukoc. Biol. 97, 455–467 (2015).
Pelaia, C. et al. Interleukin-5 in the pathophysiology of severe asthma. Front. Physiol. 10, 1514 (2019).
Kitano, T. et al. Association between absolute eosinophil count and complex aortic arch plaque in patients with acute ischemic stroke. Stroke 48, 1074–1076 (2017).
Xu, W. J. et al. Arterial and venous thromboembolism risk associated with blood eosinophils: a systematic review and meta-analysis. Anim. Model Exp. Med. 5, 470–481 (2022).
Spriewald, B. M., Ensminger, S. M., Billing, J. S., Morris, P. J. & Wood, K. J. Increased expression of transforming growth factor-beta and eosinophil infiltration is associated with the development of transplant arteriosclerosis in long-term surviving cardiac allografts. Transplantation 76, 1105–1111 (2003).
Marx, C. et al. Eosinophil–platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 134, 1859–1872 (2019).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Doring, Y., Libby, P. & Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ. Res. 126, 1228–1241 (2020).
Thakur, M. et al. NETs-induced thrombosis impacts on cardiovascular and chronic kidney disease. Circ. Res. 132, 933–949 (2023).
Riascos-Bernal, D. F. & Sibinga, N. E. Neutrophil extracellular traps in cardiac hypertrophy: a KLF2 perspective. J. Clin. Invest. 132, e156453 (2022).
Yousefi, S. et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14, 949–953 (2008).
Mukai, H. Y., Ninomiya, H., Ohtani, K., Nagasawa, T. & Abe, T. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br. J. Haematol. 90, 892–899 (1995).
Slungaard, A., Vercellotti, G. M., Tran, T., Gleich, G. J. & Key, N. S. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J. Clin. Invest. 91, 1721–1730 (1993).
Mukherjee, M., Lacy, P. & Ueki, S. Eosinophil extracellular traps and inflammatory pathologies — untangling the web! Front. Immunol. 9, 2763 (2018).
Ueki, S. et al. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr. Allergy Asthma Rep. 16, 54 (2016).
Choi, Y. et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 75, 95–103 (2020).
Olsson, I. & Venge, P. Cationic proteins of human granulocytes. I. Isolation of the cationic proteins from the granules of leukaemic myeloid cells. Scand. J. Haematol. 9, 204–214 (1972).
Gleich, G. J., Loegering, D. A. & Maldonado, J. E. Identification of a major basic protein in guinea pig eosinophil granules. J. Exp. Med. 137, 1459–1471 (1973).
Venge, P., Zetterstrom, O., Dahl, R., Roxin, L. E. & Olsson, I. Low levels of eosinophil cationic proteins in patients with asthma. Lancet 2, 373–375 (1977).
Hallgren, R., Venge, P., Cullhed, I. & Olsson, I. Blood eosinophils and eosinophil cationic protein after acute myocardial infarction or corticosteroid administration. Br. J. Haematol. 42, 147–154 (1979).
Margolis, J. R. et al. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology 137, 609–616 (1980).
Baumgart, D. et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J. Am. Coll. Cardiol. 30, 57–64 (1997).
Mintz, G. S. et al. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J. Am. Coll. Cardiol. 29, 268–274 (1997).
Tanaka, M. et al. Eosinophil count is positively correlated with coronary artery calcification. Hypertens. Res. 35, 325–328 (2012).
Hou, L. et al. White blood cell count in young adulthood and coronary artery calcification in early middle age: Coronary Artery Risk Development in Young Adults (CARDIA) study. Eur. J. Epidemiol. 28, 735–742 (2013).
Diederichsen, A. C. et al. The Danish Cardiovascular Screening Trial (DANCAVAS): study protocol for a randomized controlled trial. Trials 16, 554 (2015).
Toor, I. S. et al. Eosinophil deficiency promotes aberrant repair and adverse remodeling following acute myocardial infarction. JACC Basic Transl. Sci. 5, 665–681 (2020).
Qin, M. et al. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis 263, 82–91 (2017).
Sawada, N. et al. Circulating oxidized LDL, increased in patients with acute myocardial infarction, is accompanied by heavily modified HDL. J. Lipid Res. 61, 816–829 (2020).
Nordin Fredrikson, G., Hedblad, B., Berglund, G. & Nilsson, J. Plasma oxidized LDL: a predictor for acute myocardial infarction? J. Intern. Med. 253, 425–429 (2003).
Deng, Y. et al. Unique phenotypes of heart resident type 2 innate lymphoid cells. Front. Immunol. 11, 802 (2020).
Yu, H., Wei, Y., Dong, Y. & Chen, P. Regulation of notch signaling pathway to innate lymphoid cells in patients with acute myocardial infarction. Immunol. Invest. 52, 241–255 (2023).
Niccoli, G. et al. Eosinophil cationic protein: a new biomarker of coronary atherosclerosis. Atherosclerosis 211, 606–611 (2010).
Niccoli, G. et al. Allergic inflammation is associated with coronary instability and a worse clinical outcome after acute myocardial infarction. Circ. Cardiovasc. Interv. 8, e002554 (2015).
Xia, G. L., Wang, Y. K. & Huang, Z. Q. The correlation of serum myeloid-related protein-8/14 and eosinophil cationic protein in patients with coronary artery disease. Biomed. Res. Int. 2016, 4980251 (2016).
Guner, A. et al. Eosinophil percentage as a new prognostic marker in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Interv. Med. Appl. Sci. 11, 146–153 (2020).
Jiang, P., Wang, D. Z., Ren, Y. L., Cai, J. P. & Chen, B. X. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron. Artery Dis. 26, 101–106 (2015).
Sincer, I., Gunes, Y., Mansiroglu, A. K. & Aktas, G. Differential value of eosinophil count in acute coronary syndrome among elderly patients. Aging Male 23, 958–961 (2020).
Sasmita, B. R. et al. Leukocyte and its subtypes as predictors of short-term outcome in cardiogenic shock complicating acute myocardial infarction: a cohort study. Shock 57, 351–359 (2022).
Ye, L. et al. Combination of eosinophil percentage and high-sensitivity C-reactive protein predicts in-hospital major adverse cardiac events in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Clin. Lab. Anal. 34, e23367 (2020).
Alkhalil, M. et al. Eosinopenia as an adverse marker of clinical outcomes in patients presenting with acute myocardial infarction. Am. J. Med. 132, e827–e834 (2019).
Niccoli, G., Kharbanda, R. K., Crea, F. & Banning, A. P. No-reflow: again prevention is better than treatment. Eur. Heart J. 31, 2449–2455 (2010).
Rezkalla, S. H., Stankowski, R. V., Hanna, J. & Kloner, R. A. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc. Interv. 10, 215–223 (2017).
Mo, D. G., Wang, C. S., Liu, J. H. & Li, T. The predictive value of eosinophil levels on no-reflow in patients with STEMI following PCI: a retrospective cohort study. Sci. Rep. 12, 17862 (2022).
Toor, I. S., Jaumdally, R., Lip, G. Y., Millane, T. & Varma, C. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb. Res. 130, 607–611 (2012).
Rios-Navarro, C. et al. Characterization and implications of the dynamics of eosinophils in blood and in the infarcted myocardium after coronary reperfusion. PLoS ONE 13, e0206344 (2018).
Caforio, A. L. et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 34, 2636–2648 (2013).
Oakley, C. M. & Olsen, G. J. Eosinophilia and heart disease. Br. Heart J. 39, 233–237 (1977).
Cugno, M., Marzano, A. V., Lorini, M., Carbonelli, V. & Tedeschi, A. Enhanced tissue factor expression by blood eosinophils from patients with hypereosinophilia: a possible link with thrombosis. PLoS ONE 9, e111862 (2014).
Seguela, P. E. et al. Eosinophilic cardiac disease: molecular, clinical and imaging aspects. Arch. Cardiovasc. Dis. 108, 258–268 (2015).
Akuthota, P. & Weller, P. F. Spectrum of eosinophilic end-organ manifestations. Immunol. Allergy Clin. North. Am. 35, 403–411 (2015).
Diny, N. L. et al. Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart. Eur. J. Immunol. 46, 2749–2760 (2016).
Brambatti, M. et al. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J. Am. Coll. Cardiol. 70, 2363–2375 (2017).
Corradi, D. et al. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: insights into mechanisms of myocardial cell death. Hum. Pathol. 35, 1160–1163 (2004).
Janin, A. Eosinophilic myocarditis and fibrosis. Hum. Pathol. 36, 592–593 (2005).
Hirasawa, M., Deguchi, H., Ukimura, A. & Kitaura, Y. Immunologic interaction between infiltrating eosinophils and T lymphocytes in murine spontaneous eosinophilic myocarditis. Int. Arch. Allergy Immunol. 130, 73–81 (2003).
Prows, D. R., Klingler, A., Gibbons, W. J. Jr, Homan, S. M. & Zimmermann, N. Characterization of a mouse model of hypereosinophilia-associated heart disease. Am. J. Physiol. Heart Circ. Physiol. 317, H405–H414 (2019).
Ogbogu, P. U., Rosing, D. R. & Horne, M. K. III Cardiovascular manifestations of hypereosinophilic syndromes. Immunol. Allergy Clin. North. Am. 27, 457–475 (2007).
Ong, S. et al. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 185, 847–861 (2015).
Barin, J. G. et al. Fatal eosinophilic myocarditis develops in the absence of IFN-gamma and IL-17A. J. Immunol. 191, 4038–4047 (2013).
Diny, N. L. et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J. Exp. Med. 214, 943–957 (2017).
Zanchetti, A. Hypertension: cardiac hypertrophy as a target of antihypertensive therapy. Nat. Rev. Cardiol. 7, 66–67 (2010).
Rader, F., Sachdev, E., Arsanjani, R. & Siegel, R. J. Left ventricular hypertrophy in valvular aortic stenosis: mechanisms and clinical implications. Am. J. Med. 128, 344–352 (2015).
Tardiff, J. C. Cardiac hypertrophy: stressing out the heart. J. Clin. Invest. 116, 1467–1470 (2006).
Shimizu, I. & Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 97, 245–262 (2016).
Vural, A. & Aydin, E. The predictive value of eosinophil indices for major cardiovascular events in patients with acute decompensated HFrEF. Medicina 58, 1455 (2022).
Silva, N., Patricio, E., Bettencourt, P. & Guimaraes, J. T. Evaluation of innate immunity biomarkers on admission and at discharge from an acute heart failure episode. J. Clin. Lab. Anal. 30, 1183–1190 (2016).
Dembic, M., Hedley, P. L., Torp-Pedersen, C., Kober, L. & Christiansen, M. Pregnancy-associated plasma protein-A (PAPP-A) and the proform of the eosinophil major basic protein (ProMBP) are associated with increased risk of death in heart failure patients. Scand. J. Clin. Lab. Invest. 77, 352–357 (2017).
Go, A. S. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 285, 2370–2375 (2001).
European Heart Rhythm Association et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation — executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J. Am. Coll. Cardiol. 48, 854–906 (2006).
Falk, R. H. Atrial fibrillation. N. Engl. J. Med. 344, 1067–1078 (2001).
Lip, G. Y. Does atrial fibrillation confer a hypercoagulable state? Lancet 346, 1313–1314 (1995).
Misialek, J. R. et al. Association of white blood cell count and differential with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. PLoS ONE 10, e0136219 (2015).
Chen, P., Chen, J., Xie, X., Zhu, J. & Xia, L. Eosinophils in patients with lone atrial fibrillation. Pacing Clin. Electrophysiol. 40, 955–958 (2017).
Kecoglu, S., Demir, M., Uyan, U. & Melek, M. The effects of eosinophil on the left atrial thrombus in patients with atrial fibrillation. Clin. Appl. Thromb. Hemost. 20, 285–289 (2014).
Cavallari, I. & Patti, G. Early risk of mortality, cardiovascular events, and bleeding in patients with newly diagnosed atrial fibrillation. Eur. Heart J. Suppl. 22, L110–L113 (2020).
Owens, A. P. III & Mackman, N. Tissue factor and thrombosis: the clot starts here. Thromb. Haemost. 104, 432–439 (2010).
Leiva, O. et al. Association of thrombosis with hypereosinophilic syndrome in patients with genetic alterations. JAMA Netw. Open 4, e2119812 (2021).
Fujita, K., Ishimaru, H., Hatta, K. & Kobashi, Y. Hypereosinophilic syndrome as a cause of fatal thrombosis: two case reports with histological study. J. Thromb. Thrombolysis 40, 255–259 (2015).
Slungaard, A. & Mahoney, J. R. Jr. Thiocyanate is the major substrate for eosinophil peroxidase in physiologic fluids. Implications for cytotoxicity. J. Biol. Chem. 266, 4903–4910 (1991).
Wang, J. G. et al. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood 107, 558–565 (2006).
Maruyama, I., Bell, C. E. & Majerus, P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J. Cell Biol. 101, 363–371 (1985).
Dittman, W. A. & Majerus, P. W. Structure and function of thrombomodulin: a natural anticoagulant. Blood 75, 329–336 (1990).
Esmon, C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J. Biol. Chem. 264, 4743–4746 (1989).
Esmon, C. T. Molecular events that control the protein C anticoagulant pathway. Thromb. Haemost. 70, 29–35 (1993).
Takano, S. Role of 5-hydroxytryptamine in platelet thrombus formation and mechanisms of inhibition of thrombus formation by 5-hydroxytryptamine2A antagonists in rabbits. Arch. Int. Pharmacodyn. Ther. 330, 297–308 (1995).
Rohrbach, M. S., Wheatley, C. L., Slifman, N. R. & Gleich, G. J. Activation of platelets by eosinophil granule proteins. J. Exp. Med. 172, 1271–1274 (1990).
Cargill, D. I., Cohen, D. S., Van Valen, R. G., Klimek, J. J. & Levin, R. P. Aggregation, release and desensitization induced in platelets from five species by platelet activating factor (PAF). Thromb. Haemost. 49, 204–207 (1983).
Shah, S. A., Page, C. P. & Pitchford, S. C. Platelet–eosinophil interactions as a potential therapeutic target in allergic inflammation and asthma. Front. Med. 4, 129 (2017).
Sakai, T. et al. Eosinophils may be involved in thrombus growth in acute coronary syndrome. Int. Heart J. 50, 267–277 (2009).
Yamaji, K. et al. Association of localized hypersensitivity and in-stent neoatherosclerosis with the very late drug-eluting stent thrombosis. PLoS ONE 9, e113870 (2014).
Riegger, J. et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur. Heart J. 37, 1538–1549 (2016).
Mansiroglu, A. K., Sincer, I., Cosgun, M. & Gunes, Y. Dating thrombus organization with eosinophil counts in deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 9, 874–880 (2021).
Kulahcioglu, S. et al. Eosinophil-to-monocyte ratio as a candidate for a novel prognostic marker in acute pulmonary embolism: is it a consumptive mechanism? Anatol. J. Cardiol. 26, 717–724 (2022).
Scheitz, J. F., Nolte, C. H., Doehner, W., Hachinski, V. & Endres, M. Stroke–heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 17, 1109–1120 (2018).
Lo, E. H., Dalkara, T. & Moskowitz, M. A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4, 399–415 (2003).
Palasubramaniam, J., Wang, X. & Peter, K. Myocardial infarction — from atherosclerosis to thrombosis. Arterioscler. Thromb. Vasc. Biol. 39, e176–e185 (2019).
Heusch, G. & Gersh, B. J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 38, 774–784 (2017).
Hori, Y. S., Kodera, S., Sato, Y. & Shiojiri, T. Eosinopenia as a predictive factor of the short-term risk of mortality and infection after acute cerebral infarction. J. Stroke Cerebrovasc. Dis. 25, 1307–1312 (2016).
Juceviciute, N., Mikuzis, P. & Balnyte, R. Absolute blood eosinophil count could be a potential biomarker for predicting haemorrhagic transformation after intravenous thrombolysis for acute ischaemic stroke. BMC Neurol. 19, 127 (2019).
Yu, S. et al. Eosinophil-to-monocyte ratio is a potential biomarker in the prediction of functional outcome among patients with acute ischemic stroke. BMC Neurosci. 22, 8 (2021).
Yang, D. et al. Dynamic decrease in eosinophil after intravenous thrombolysis predicts poor prognosis of acute ischemic stroke: a longitudinal study. Front. Immunol. 12, 709289 (2021).
Chen, Y. et al. Eosinophil-to-monocyte ratio is a potential predictor of prognosis in acute ischemic stroke patients after intravenous thrombolysis. Clin. Interv. Aging 16, 853–862 (2021).
Fan, L., Gui, L., Chai, E. Q. & Wei, C. J. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J. Clin. Lab. Anal. 32, e22244 (2018).
Gunes, M. Is neutrophil/eosinophil ratio at admission a prognostic marker for in-hospital mortality of acute ischemic stroke? J. Stroke Cerebrovasc. Dis. 29, 104999 (2020).
Sundstrom, J. et al. Eosinophil cationic protein, carotid plaque, and incidence of stroke. Stroke 48, 2686–2692 (2017).
Watts, S. W., Flood, E. D., Garver, H., Fink, G. D. & Roccabianca, S. A new function for perivascular adipose tissue (PVAT): assistance of arterial stress relaxation. Sci. Rep. 10, 1807 (2020).
Ailawadi, G. et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 138, 1392–1399 (2009).
Crosas-Molist, E. et al. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 35, 960–972 (2015).
Withers, S. B. et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci. Rep. 7, 44571 (2017).
Langenskiold, M., Smidfelt, K., Nordanstig, J., Bergstrom, G. & Tivesten, A. Leukocyte subsets and abdominal aortic aneurysms detected by screening in men. J. Intern. Med. 288, 345–355 (2020).
Harris, C., Croce, B. & Cao, C. Type A aortic dissection. Ann. Cardiothorac. Surg. 5, 256 (2016).
Harris, C. G., Croce, B. & Tian, D. H. Type B aortic dissection. Ann. Cardiothorac. Surg. 3, 339 (2014).
Shao, Y. et al. Impacts of eosinophil percentage on prognosis acute type A aortic dissection patients. BMC Cardiovasc. Disord. 22, 146 (2022).
Qin, X. et al. The role of peripheral blood eosinophil counts in acute Stanford type A aortic dissection patients. Front. Surg. 9, 969995 (2022).
Zhao, K. et al. Peripheral eosinophil count is associated with the prognosis of patients with type B aortic dissection undergoing endovascular aortic repair: a retrospective cohort study. J. Am. Heart Assoc. 11, e027339 (2022).
Miyata, J. et al. 12/15-Lipoxygenase regulates IL-33-induced eosinophilic airway inflammation in mice. Front. Immunol. 12, 687192 (2021).
Miyata, J. et al. Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases. Prostaglandins Other Lipid Mediat. 150, 106477 (2020).
Masterson, J. C. et al. CCR3 blockade attenuates eosinophilic ileitis and associated remodeling. Am. J. Pathol. 179, 2302–2314 (2011).
Miyata, J. et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 131, 353–360 (2013).
Metcalfe, D. D. et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 9, 7 (2016).
Johansson, M. W. Activation states of blood eosinophils in asthma. Clin. Exp. Allergy 44, 482–498 (2014).
Johansson, M. W. et al. Platelet activation, P-selectin, and eosinophil beta1-integrin activation in asthma. Am. J. Respir. Crit. Care Med. 185, 498–507 (2012).
Lingblom, C., Andersson, J., Andersson, K. & Wenneras, C. Regulatory eosinophils suppress T cells partly through galectin-10. J. Immunol. 198, 4672–4681 (2017).
Pincus, S. H., Schooley, W. R., DiNapoli, A. M. & Broder, S. Metabolic heterogeneity of eosinophils from normal and hypereosinophilic patients. Blood 58, 1175–1181 (1981).
Li, W., McIntyre, T. M. & Silverstein, R. L. Ferric chloride-induced murine carotid arterial injury: a model of redox pathology. Redox Biol. 1, 50–55 (2013).
Uderhardt, S. et al. Enzymatic lipid oxidation by eosinophils propagates coagulation, hemostasis, and thrombotic disease. J. Exp. Med. 214, 2121–2138 (2017).
Blankenhorn, D. H. & Stern, D. Calcification of the coronary arteries. Am. J. Roentgenol. Radium Ther. Nucl. Med. 81, 772–777 (1959).
Libby, P. Murine ‘model’ monotheism: an iconoclast at the altar of mouse. Circ. Res. 117, 921–925 (2015).
Acknowledgements
The authors thank C. Swallom (Brigham and Women’s Hospital, Boston, MA, USA) for editorial assistance with the manuscript before submission. The authors received support from the Hainan Province Science and Technology special fund (ZDYF2020214 to J.G.); the National Natural Science Foundation of China (82300278 to J.X., 81770487, 91939107 and 82170440 to J.G. and 82170234 to C.Y.); the National Natural Science Foundation of China Incubation Project of Guangdong Provincial People’s Hospital (KY0120220041 to J.X.); the National Science Fund for Distinguished Young Scholars of Hainan Medical University (JBGS202104 to J.G.); the National Heart, Lung and Blood Institute (HL151627, HL157073, HL166538 and HL170000 to G.-P.S. and HL134892 and HL163099 to P.L.) and the National Institute of Neurological Disorders and Stroke (AG063839 to G.-P.S.).
Author information
Authors and Affiliations
Contributions
J.X., J.G., T.L., Z.M., J.Z. and G.-P.S. researched data for the article. J.X., J.G. and G.-P.S. wrote the manuscript. J.X., J.G., C.Y., P.L., J.Z. and G.-P.S. substantially contributed to discussion of content. J.X., J.G., P.L. and G.-P.S. reviewed or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Daniela Cihakova, Norbert Gerdes, Sumanth Prabhu, Konstantin Stark and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Guo, J., Liu, T. et al. Differential roles of eosinophils in cardiovascular disease. Nat Rev Cardiol (2024). https://doi.org/10.1038/s41569-024-01071-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-024-01071-5