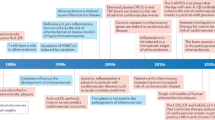
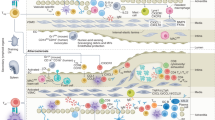
Abstract
Autoimmune diseases are associated with a dramatically increased risk of atherosclerotic cardiovascular disease and its clinical manifestations. The increased risk is consistent with the notion that atherogenesis is modulated by both protective and disease-promoting immune mechanisms. Notably, traditional cardiovascular risk factors such as dyslipidaemia and hypertension alone do not explain the increased risk of cardiovascular disease associated with autoimmune diseases. Several mechanisms have been implicated in mediating the autoimmunity-associated cardiovascular risk, either directly or by modulating the effect of other risk factors in a complex interplay. Aberrant leukocyte function and pro-inflammatory cytokines are central to both disease entities, resulting in vascular dysfunction, impaired resolution of inflammation and promotion of chronic inflammation. Similarly, loss of tolerance to self-antigens and the generation of autoantibodies are key features of autoimmunity but are also implicated in the maladaptive inflammatory response during atherosclerotic cardiovascular disease. Therefore, immunomodulatory therapies are potential efficacious interventions to directly reduce the risk of cardiovascular disease, and biomarkers of autoimmune disease activity could be relevant tools to stratify patients with autoimmunity according to their cardiovascular risk. In this Review, we discuss the pathophysiological aspects of the increased cardiovascular risk associated with autoimmunity and highlight the many open questions that need to be answered to develop novel therapies that specifically address this unmet clinical need.
Key points
-
Autoimmune diseases, including systemic lupus erythematosus and rheumatoid arthritis, are associated with an increased risk of atherosclerotic cardiovascular disease and cardiovascular death.
-
Epidemiological data in large cohorts of patients and experimental evidence in preclinical models have clearly established the link between autoimmunity and atherosclerosis.
-
Both shared and distinct mechanisms increase the risk of atherosclerosis in patients with systemic lupus erythematosus, rheumatoid arthritis or other connective-tissue autoimmune diseases.
-
Endothelial dysfunction, increased cytokine signalling (IL-1β, IL-6 and tumour necrosis factor) and leukocyte activation (monocytes, macrophages and neutrophils), and aberrant T cell and B cell functions are key mechanisms for accelerated atherosclerosis in patients with autoimmune diseases.
-
Knowledge about the functional role of classic autoimmune disease-associated autoantibodies in promoting atherosclerosis is limited.
-
Anti-inflammatory therapies that are being evaluated for atherosclerotic cardiovascular disease might represent important opportunities for reducing the risk of atherosclerosis particularly in patients with autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ridker, P. M. et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet 401, 1293–1301 (2023).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Fiolet, A. T. L. et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur. Heart J. 42, 2765–2775 (2021).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Byrne, R. A. et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur. Heart J. Acute Cardiovasc 44, 3720–3826 (2023).
Ridker, P. M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ. Res. 118, 145–156 (2016).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Porsch, F., Mallat, Z. & Binder, C. J. Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies. Cardiovasc. Res. 117, 2544–2562 (2021).
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. 22, 251–265 (2022).
Mallat, Z. & Binder, C. J. The why and how of adaptive immune responses in ischemic cardiovascular disease. Nat. Cardiovasc. Res. 1, 431–444 (2022).
Conrad, N. et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 400, 733–743 (2022).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 18001 (2018).
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2, 16039 (2016).
Skaggs, B. J., Hahn, B. H. & McMahon, M. Accelerated atherosclerosis in patients with SLE – mechanisms and management. Nat. Rev. Rheumatol. 8, 214–223 (2012).
Ambler, W. G. & Kaplan, M. J. Vascular damage in systemic lupus erythematosus. Nat. Rev. Nephrol. 20, 251–265 (2024).
Weber, B. N., Giles, J. T. & Liao, K. P. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat. Rev. Rheumatol. 19, 417–428 (2023).
Forte, F. et al. Association of systemic lupus erythematosus with peripheral arterial disease: a meta-analysis of literature studies. Rheumatology 59, 3181–3192 (2020).
Restivo, V. et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun. Rev. 21, 102925 (2022).
Yafasova, A. et al. Long-term cardiovascular outcomes in systemic lupus erythematosus. J. Am. Coll. Cardiol. 77, 1717–1727 (2021).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Sparks, J. A. et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the Nurses’ Health Study. Arthritis Care Res 68, 753–762 (2016).
Bartoloni, E. et al. Cardiovascular disease risk burden in primary Sjögren’s syndrome: results of a population-based multicentre cohort study. J. Intern. Med. 278, 185–192 (2015).
Butt, S. A. et al. Cardiovascular manifestations of systemic sclerosis: a Danish nationwide cohort study. J. Am. Heart Assoc. 8, e013405 (2019).
Kiani, A. N. Coronary calcification in SLE: comparison with the Multi-Ethnic Study of Atherosclerosis. Rheumatology 54, 1976–1981 (2015).
Carlucci, P. M. et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 3, e99276 (2018).
Agca, R. et al. Arterial wall inflammation is increased in rheumatoid arthritis compared with osteoarthritis, as a marker of early atherosclerosis. Rheumatology 60, 3360–3368 (2021).
Geraldino-Pardilla, L. et al. Arterial inflammation detected with 18F-fluorodeoxyglucose-positron emission tomography in rheumatoid arthritis. Arthritis Rheumatol. 70, 30–39 (2018).
Hansen, P. R., Feineis, M. & Abdulla, J. Rheumatoid arthritis patients have higher prevalence and burden of asymptomatic coronary artery disease assessed by coronary computed tomography: a systematic literature review and meta-analysis. Eur. J. Intern. Med. 62, 72–79 (2019).
Tyrrell, P. N. et al. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 30, 1014–1026 (2010).
Willeit, P. et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation 142, 621–642 (2020).
Gautier, E. L. et al. Enhanced immune system activation and arterial inflammation accelerates atherosclerosis in lupus-prone mice. Arterioscler. Thromb. Vasc. Biol. 27, 1625–1631 (2007).
Aprahamian, T. et al. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J. Exp. Med. 199, 1121–1131 (2004).
Ma, Z. et al. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin. Immunol. 127, 168–175 (2008).
Feng, X. et al. ApoE−/−Fas−/− C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J. Lipid Res. 48, 794–805 (2007).
Lewis, M. J. et al. Distinct roles for complement in glomerulonephritis and atherosclerosis revealed in mice with a combination of lupus and hyperlipidemia. Arthritis Rheum. 64, 2707–2718 (2012).
Stanic, A. K. et al. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 103, 7018–7023 (2006).
Braun, N. A., Wade, N. S., Wakeland, E. K. & Major, A. S. Accelerated atherosclerosis is independent of feeding high fat diet in systemic lupus erythematosus-susceptible LDLr−/− mice. Lupus 17, 1070–1078 (2008).
Wilhelm, A. J., Rhoads, J. P., Wade, N. S. & Major, A. S. Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr−/− mice. Ann. Rheum. Dis. 74, 778–785 (2015).
Postigo, J. et al. Exacerbation of type II collagen-induced arthritis in apolipoprotein E-deficient mice in association with the expansion of Th1 and Th17 cells. Arthritis Rheum. 63, 971–980 (2011).
Shi, N. et al. Protective effect of hydroxychloroquine on rheumatoid arthritis-associated atherosclerosis. Anim. Models Exp. Med. 2, 98–106 (2019).
Santiago-Raber, M.-L. et al. Atherosclerotic plaque vulnerability is increased in mouse model of lupus. Sci. Rep. 10, 18324 (2020).
Centa, M. et al. Acute loss of apolipoprotein E triggers an autoimmune response that accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 38, e145–e158 (2018).
Hutchinson, M. A. et al. Auto-antibody production during experimental atherosclerosis in ApoE−/− mice. Front. Immunol. 12, 695220 (2021).
Ryu, H. et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 19, 583–593 (2018).
Afek, A. et al. Enhancement of atherosclerosis in beta-2-glycoprotein I-immunized apolipoprotein E-deficient mice. Pathobiology 67, 19–25 (1999).
Wang, X. et al. Anti-β2GPI antibodies enhance atherosclerosis in ApoE-deficient mice. Biochem. Biophys. Res. Commun. 512, 72–78 (2019).
Nicolo, D., Goldman, B. I. & Monestier, M. Reduction of atherosclerosis in low-density lipoprotein receptor-deficient mice by passive administration of antiphospholipid antibody. Arthritis Rheum. 48, 2974–2978 (2003).
Rose, S. et al. A novel mouse model that develops spontaneous arthritis and is predisposed towards atherosclerosis. Ann. Rheum. Dis. 72, 89–95 (2013).
Archer, A. M. et al. ApoE deficiency exacerbates the development and sustainment of a semi-chronic K/BxN serum transfer-induced arthritis model. J. Transl. Med. 14, 170 (2016).
Dragoljevic, D. et al. Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis. Eur. Heart J. 39, 2158–2167 (2018).
Timmis, A. et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur. Heart J. 43, 716–799 (2022).
Hermansen, M.-L. et al. Atherosclerosis and renal disease involvement in patients with systemic lupus erythematosus: a cross-sectional cohort study. Rheumatology 57, 1964–1971 (2018).
Robinson, G., Pineda-Torra, I., Ciurtin, C. & Jury, E. C. Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J. Clin. Invest. 132, e148552 (2022).
Takvorian, S. U., Merola, J. F. & Costenbader, K. H. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus 23, 537–544 (2014).
Maisha, J. A., El-Gabalawy, H. S. & O’Neil, L. J. Modifiable risk factors linked to the development of rheumatoid arthritis: evidence, immunological mechanisms and prevention. Front. Immunol. 14, 1221125 (2023).
Szabó, M. Z., Szodoray, P. & Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 65, 543–550 (2017).
Purmalek, M. M. et al. Association of lipoprotein subfractions and glycoprotein acetylation with coronary plaque burden in SLE. Lupus Sci. Med. 6, e000332 (2019).
Ramos-Casals, M. et al. High prevalence of serum metabolic alterations in primary Sjögren’s syndrome: influence on clinical and immunological expression. J. Rheumatol. 34, 754–761 (2007).
Kronbichler, A., Leierer, J., Gauckler, P. & Shin, J. I. Comorbidities in ANCA-associated vasculitis. Rheumatology 59, iii79–iii83 (2020).
McMahon, M. et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 54, 2541–2549 (2006).
Charles-Schoeman, C. et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 60, 2870–2879 (2009).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66, 2532–2544 (2014).
Charles-Schoeman, C. et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin. Arthritis Rheum. 46, 71–80 (2016).
Yan, J. et al. Dyslipidemia in rheumatoid arthritis: the possible mechanisms. Front. Immunol. 14, 1254753 (2023).
Turesson, C., Bergström, U., Pikwer, M., Nilsson, J.-Å. & Jacobsson, L. T. High serum cholesterol predicts rheumatoid arthritis in women, but not in men: a prospective study. Arthritis Res. Ther. 17, 284 (2015).
Wang, M. et al. The causal relationship between blood lipids and systemic lupus erythematosus risk: a bidirectional two-sample Mendelian randomization study. Front. Genet. 13, 858653 (2022).
Kawai, V. K. et al. Pleiotropy of systemic lupus erythematosus risk alleles and cardiometabolic disorders: a phenome-wide association study and inverse-variance weighted meta-analysis. Lupus 30, 1264–1272 (2021).
Schloss, M. J., Swirski, F. K. & Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis and innate immunity. Circ. Res. 126, 1242–1259 (2020).
Jury, E. C., Isenberg, D. A., Mauri, C. & Ehrenstein, M. R. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J. Immunol. 177, 7416–7422 (2006).
Krishnan, S. et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 172, 7821–7831 (2004).
Baardman, J. & Lutgens, E. Regulatory T cell metabolism in atherosclerosis. Metabolites 10, 279 (2020).
Maganto-García, E., Tarrio, M. L., Grabie, N., Bu, D. & Lichtman, A. H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124, 185–195 (2011).
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Klingenberg, R. et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 123, 1323–1334 (2013).
Wang, Z. et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat. Cardiovasc. Res. 2, 290–306 (2023).
Khan, A., Roy, P. & Ley, K. Breaking tolerance: the autoimmune aspect of atherosclerosis. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-024-01010-y (2024).
Ito, A. et al. Cholesterol accumulation in CD11c+ immune cells is a causal and targetable factor in autoimmune disease. Immunity 45, 1311–1326 (2016).
Westerterp, M. et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 25, 1294–1304.e6 (2017).
Rahman, P., Aguero, S., Gladman, D. D., Hallett, D. & Urowitz, M. B. Vascular events in hypertensive patients with systemic lupus erythematosus. Lupus 9, 672–675 (2000).
Bartoloni, E., Alunno, A. & Gerli, R. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat. Rev. Cardiol. 15, 33–44 (2018).
Costello, R. E., Yimer, B. B., Roads, P., Jani, M. & Dixon, W. G. Glucocorticoid use is associated with an increased risk of hypertension. Rheumatology 60, 132–139 (2021).
Mathis, K. W. et al. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 64, 792–800 (2014).
McClung, D. M., Kalusche, W. J., Jones, K. E., Ryan, M. J. & Taylor, E. B. Hypertension and endothelial dysfunction in the pristane model of systemic lupus erythematosus. Physiol. Rep. 9, e14734 (2021).
Zhang, K. et al. Rheumatoid arthritis and the risk of major cardiometabolic diseases: a Mendelian randomization study. Scand. J. Rheumatol. 52, 335–341 (2023).
Rohm, T. V., Meier, D. T., Olefsky, J. M. & Donath, M. Y. Inflammation in obesity, diabetes, and related disorders. Immunity 55, 31–55 (2022).
Haase, C. L., Tybjærg-Hansen, A., Nordestgaard, B. G. & Frikke-Schmidt, R. HDL cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes 64, 3328–3333 (2015).
Mellor, D. D. et al. Association between lipids and apolipoproteins on type 2 diabetes risk; moderating effects of gender and polymorphisms; the ATTICA study. Nutr. Metab. Cardiovasc. Dis. 30, 788–795 (2020).
Peng, J. et al. Association between dyslipidemia and risk of type 2 diabetes mellitus in middle-aged and older Chinese adults: a secondary analysis of a nationwide cohort. BMJ Open 11, e042821 (2021).
de Resende Guimarães, M. F. B. et al. High prevalence of obesity in rheumatoid arthritis patients: association with disease activity. hypertension, dyslipidemia and diabetes, a multi-center study. Adv. Rheumatol. 59, 44 (2019).
Tamargo, I. A., Baek, K. I., Kim, Y., Park, C. & Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 20, 738–753 (2023).
Gimbrone, M. A. & García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Bordy, R. et al. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat. Rev. Rheumatol. 14, 404–420 (2018).
Matucci-Cerinic, M., Kahaleh, B. & Wigley, F. M. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 65, 1953–1962 (2013).
Weber, B. N. et al. Coronary microvascular dysfunction in systemic lupus erythematosus. J. Am. Heart Assoc. 10, e018555 (2021).
Conrad, N. et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet 401, 1878–1890 (2023).
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Prim. 1, 15002 (2015).
Libby, P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 70, 2278–2289 (2017).
Chen, H.-J., Tas, S. W. & de Winther, M. P. J. Type-I interferons in atherosclerosis. J. Exp. Med. 217, e20190459 (2020).
Urschel, K. & Cicha, I. TNF-α in the cardiovascular system: from physiology to therapy. Int. J. Interferon Cytokine Mediat. Res. 7, 9–25 (2015).
Buie, J. J., Renaud, L. L., Muise-Helmericks, R. & Oates, J. C. IFN-α negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. J. Immunol. 199, 1979–1988 (2017).
Akhmedov, A. et al. TNFα induces endothelial dysfunction in rheumatoid arthritis via LOX-1 and arginase 2: reversal by monoclonal TNFα antibodies. Cardiovasc. Res. 118, 254–266 (2022).
Mak, A. et al. Endothelial dysfunction in systemic lupus erythematosus – a case-control study and an updated meta-analysis and meta-regression. Sci. Rep. 7, 7320 (2017).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Denny, M. F. et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M. J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015).
Rajagopalan, S. et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood 103, 3677–3683 (2004).
Lee, P. Y. et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 56, 3759–3769 (2007).
Pieterse, E. et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 37, 1371–1379 (2017).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Schreiber, A. et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA 114, E9618–E9625 (2017).
Nakazawa, D., Masuda, S., Tomaru, U. & Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 15, 91–101 (2019).
Lood, C. et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 116, 1951–1957 (2010).
Nhek, S. et al. Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 707–716 (2017).
Maugeri, N. et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci. Transl. Med. 10, eaao3089 (2018).
Legendre, P., Régent, A., Thiebault, M. & Mouthon, L. Anti-endothelial cell antibodies in vasculitis: a systematic review. Autoimmun. Rev. 16, 146–153 (2017).
Truchetet, M. E., Brembilla, N. C. & Chizzolini, C. Current concepts on the pathogenesis of systemic sclerosis. Clin. Rev. Allergy Immunol. 64, 262–283 (2023).
Almanzar, G. et al. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J. Autoimmun. 39, 441–450 (2012).
Crane, E. D. et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight 3, e99363 (2018).
Ait-Oufella, H., Taleb, S., Mallat, Z. & Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 969–979 (2011).
Ait-Oufella, H., Libby, P. & Tedgui, A. Anticytokine immune therapy and atherothrombotic cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 39, 1510–1519 (2019).
Shin, J. I. et al. Inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J. Autoimmun. 103, 102299 (2019).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021).
Svensson, E. C. et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7, 521–528 (2022).
David, C. et al. Clonal haematopoiesis of indeterminate potential and cardiovascular events in systemic lupus erythematosus (HEMATOPLUS study). Rheumatology 61, 4355–4363 (2022).
Broderick, L. & Hoffman, H. M. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 18, 448–463 (2022).
Clark, W., Jobanputra, P., Barton, P. & Burls, A. The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis. Health Technol. Assess. 8, 18 (2004).
Schiff, M. H. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann. Rheum. Dis. 59, i103–i108 (2000).
Eastgate, J. A. et al. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet 2, 706–709 (1988).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 128, 1728–1746 (2021).
van der Harst, P. & Verweij, N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 122, 433–443 (2018).
Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 379, 1214–1224 (2012).
Sarwar, N. et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 379, 1205–1213 (2012).
Rosa, M. et al. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. NPJ Genom. Med 4, 23 (2019).
Bick, A. G. et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 141, 124–131 (2020).
Ridker, P. M. et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 39, 3499–3507 (2018).
Romano, M. et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 (1997).
Alsaffar, H., Martino, N., Garrett, J. P. & Adam, A. P. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am. J. Physiol. Cell Physiol. 314, C589–C602 (2018).
Neumann, F.-J. et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 17, 3399–3405 (1997).
Holt, I., Cooper, R. G. & Hopkins, S. J. Relationships between local inflammation, interleukin-6 concentration and the acute phase protein response in arthritis patients. Eur. J. Clin. Invest. 21, 479–484 (1991).
Marczynski, P. et al. Vascular inflammation and dysfunction in lupus-prone mice-IL-6 as mediator of disease initiation. Int. J. Mol. Sci. 22, 2291 (2021).
Weber, B. et al. Relationship between risk of atherosclerotic cardiovascular disease, inflammation, and coronary microvascular dysfunction in rheumatoid arthritis. J. Am. Heart Assoc. 11, e025467 (2022).
Bacchiega, B. C. et al. Interleukin 6 inhibition and coronary artery disease in a high‐risk population: a prospective community‐based clinical study. J. Am. Heart Assoc. 6, e005038 (2017).
Protogerou, A. D. et al. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 219, 734–736 (2011).
Souto, A. et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 67, 117–127 (2015).
McInnes, I. B. et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 74, 694–702 (2015).
Pierini, F. S. et al. Effect of tocilizumab on LDL and HDL characteristics in patients with rheumatoid arthritis. an observational study. Rheumatol. Ther. 8, 803–815 (2021).
Giles, J. T. et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 72, 31–40 (2020).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT05021835 (2024).
van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Siegmund, D. & Wajant, H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat. Rev. Rheumatol. 19, 576–591 (2023).
Weckerle, C. E. et al. Large scale analysis of tumor necrosis factor ɑ levels in systemic lupus erythematosus. Arthritis Rheum. 64, 2947–2952 (2012).
Rho, Y. H. et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 61, 1580–1585 (2009).
Del Porto, F. et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology 46, 1111–1115 (2007).
Papamichail, G. V. et al. The effects of biologic agents on cardiovascular risk factors and atherosclerosis in rheumatoid arthritis patients: a prospective observational study. Heart Vessels 37, 2128–2136 (2022).
Jacobsson, L. T. H. et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 32, 1213–1218 (2005).
Dixon, W. G. et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor ɑ therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 56, 2905–2912 (2007).
Barnabe, C., Martin, B.-J. & Ghali, W. A. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 63, 522–529 (2011).
Roubille, C. et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 74, 480–489 (2015).
McKellar, G. E., McCarey, D. W., Sattar, N. & McInnes, I. B. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 6, 410–417 (2009).
Ridker, P. M. et al. Elevation of tumor necrosis factor-ɑ and increased risk of recurrent coronary events after myocardial infarction. Circulation 101, 2149–2153 (2000).
Chung, E. S. et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-ɑ, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Mann, D. L. et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109, 1594–1602 (2004).
Solomon, D. H. et al. Reducing cardiovascular risk with immunomodulators: a randomised active comparator trial among patients with rheumatoid arthritis. Ann. Rheum. Dis. 82, 324–330 (2023).
Goossens, P. et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 12, 142–153 (2010).
Li, J. et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-α and atherosclerosis in lupus. Arthritis Rheum. 63, 492–502 (2011).
Boshuizen, M. C. S. et al. Interferon-β promotes macrophage foam cell formation by altering both cholesterol influx and efflux mechanisms. Cytokine 77, 220–226 (2016).
Pulliam, L., Calosing, C., Sun, B., Grunfeld, C. & Rempel, H. Monocyte activation from interferon-α in HIV infection increases acetylated LDL uptake and ROS production. J. Interferon Cytokine Res. 34, 822–828 (2014).
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Kim, K. et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ. Res. 123, 1127–1142 (2018).
Zernecke, A. et al. Integrated single-cell analysis-based classification of vascular mononuclear phagocytes in mouse and human atherosclerosis. Cardiovasc. Res. 119, 1676–1689 (2023).
King, K. R. et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487 (2017).
Lin, J.-D. et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4, e124574 (2019).
Park, S. H. et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 18, 1104–1116 (2017).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Reboldi, A. et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Lövgren, T., Eloranta, M.-L., Båve, U., Alm, G. V. & Rönnblom, L. Induction of interferon-ɑ production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Barrat, F. J., Meeker, T., Chan, J. H., Guiducci, C. & Coffman, R. L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 37, 3582–3586 (2007).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Blanco, L. P. et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 73, 2282–2292 (2021).
Bellini, R., Bonacina, F. & Norata, G. D. Crosstalk between dendritic cells and T lymphocytes during atherogenesis: focus on antigen presentation and break of tolerance. Front. Cardiovasc. Med. 9, 934314 (2022).
Nagahama, M. et al. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 33, 85–94 (2001).
Duffau, P. et al. Platelet CD154 potentiates interferon-ɑ secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63 (2010).
Massberg, S. et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 196, 887–896 (2002).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67 (2003).
Barrett, T. J. et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci. Transl. Med. 11, eaax0481 (2019).
Crow, M. K. & Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res Ther. 5, 279 (2003).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Eloranta, M.-L. et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann. Rheum. Dis. 69, 1396–1402 (2010).
van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014).
Gottenberg, J.-E. et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl Acad. Sci. USA 103, 2770–2775 (2006).
Muskardin, T. L. W. & Niewold, T. B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 14, 214–228 (2018).
Somers, E. C. et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PloS ONE 7, e37000 (2012).
Rogacev, K. S. et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 60, 1512–1520 (2012).
Lioté, F., Boval-Boizard, B., Weill, D., Kuntz, D. & Wautier, J. L. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin. Exp. Immunol. 106, 13–19 (1996).
Rossol, M., Kraus, S., Pierer, M., Baerwald, C. & Wagner, U. The CD14brightCD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 64, 671–677 (2012).
Korman, B. D. et al. Inflammatory expression profiles in monocyte-to-macrophage differentiation in patients with systemic lupus erythematosus and relationship with atherosclerosis. Arthritis Res. Ther. 16, R147 (2014).
O’Gorman, W. E. et al. Single-cell systems-level analysis of human Toll-like receptor activation defines a chemokine signature in patients with systemic lupus erythematosus. J. Allergy Clin. Immunol. 136, 1326–1336 (2015).
Shi, L. et al. Monocyte enhancers are highly altered in systemic lupus erythematosus. Epigenomics 7, 921–935 (2015).
Mikołajczyk, T. P. et al. Heterogeneity of peripheral blood monocytes, endothelial dysfunction and subclinical atherosclerosis in patients with systemic lupus erythematosus. Lupus 25, 18–27 (2016).
López, P. et al. Low-density granulocytes and monocytes as biomarkers of cardiovascular risk in systemic lupus erythematosus. Rheumatology 59, 1752–1764 (2020).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Combadière, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Adamstein, N. H. et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur. Heart J. 42, 896–903 (2021).
Nurmohamed, N. S. et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur. Heart J. 43, 1569–1577 (2022).
Luo, J., Thomassen, J. Q., Nordestgaard, B. G., Tybjærg-Hansen, A. & Frikke-Schmidt, R. Neutrophil counts and cardiovascular disease. Eur. Heart J. 44, 4953–4964 (2023).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217 (2008).
Drechsler, M., Megens, R. T. A., van Zandvoort, M., Weber, C. & Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010).
Delporte, C. et al. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J. Lipid Res. 55, 747–757 (2014).
Döring, Y. et al. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res. 110, 1052–1056 (2012).
Nakamura, Y. et al. Increased LL37 in psoriasis and other inflammatory disorders promotes LDL uptake and atherosclerosis. J. Clin. Invest. 134, e172578 (2024).
Knight, J. S. et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114, 947–956 (2014).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Silvestre-Roig, C. et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019).
Mangold, A. et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 116, 1182–1192 (2015).
Libby, P., Pasterkamp, G., Crea, F. & Jang, I.-K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 124, 150–160 (2019).
Gómez-Moreno, D., Adrover, J. M. & Hidalgo, A. Neutrophils as effectors of vascular inflammation. Eur. J. Clin. Invest. 48, e12940 (2018).
Franck, G. et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice. Circ. Res. 121, 31–42 (2017).
Franck, G. et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury. Circ. Res. 123, 33–42 (2018).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA 107, 15880–15885 (2010).
Folco, E. J. et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 38, 1901–1912 (2018).
Ferrante, G. et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes. Circulation 122, 2505–2513 (2010).
Apel, F., Zychlinsky, A. & Kenny, E. F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 14, 467–475 (2018).
Wigerblad, G. & Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 23, 274–288 (2023).
Wipke, B. T. & Allen, P. M. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167, 1601–1608 (2001).
Grieshaber-Bouyer, R. et al. Ageing and interferon gamma response drive the phenotype of neutrophils in the inflamed joint. Ann. Rheum. Dis. 81, 805–814 (2022).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Döring, Y. et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683 (2012).
Rahman, S. et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann. Rheum. Dis. 78, 957–966 (2019).
Kiss, M. G. & Binder, C. J. The multifaceted impact of complement on atherosclerosis. Atherosclerosis 351, 29–40 (2022).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Seifert, P. S., Hugo, F., Hansson, G. K. & Bhakdi, S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab. Invest. 60, 747–754 (1989).
Vlaicu, R., Rus, H. G., Niculescu, F. & Cristea, A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis 55, 35–50 (1985).
Kiss, M. G. et al. Cell-autonomous regulation of complement C3 by factor H limits macrophage efferocytosis and exacerbates atherosclerosis. Immunity 56, 1809–1824.e10 (2023).
Truedsson, L., Bengtsson, A. A. & Sturfelt, G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40, 560–566 (2007).
Stojan, G. & Petri, M. Anti-C1q in systemic lupus erythematosus. Lupus 25, 873–877 (2016).
Dragon-Durey, M.-A., Blanc, C., Marinozzi, M. C., van Schaarenburg, R. A. & Trouw, L. A. Autoantibodies against complement components and functional consequences. Mol. Immunol. 56, 213–221 (2013).
Santer, D. M. et al. C1q deficiency leads to the defective suppression of IFN-ɑ in response to nucleoprotein containing immune complexes. J. Immunol. 185, 4738–4749 (2010).
Coss, S. L. et al. The complement system and human autoimmune diseases. J. Autoimmun. 137, 102979 (2023).
Aringer, M. et al. 2019 EULAR/ACR classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 71, 1400–1412 (2019).
Chen, M., Jayne, D. R. W. & Zhao, M.-H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat. Rev. Nephrol. 13, 359–367 (2017).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 (2006).
Sharma, M. et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ. Res. 127, 335–353 (2020).
Rosetti, F., Madera-Salcedo, I. K., Rodríguez-Rodríguez, N. & Crispín, J. C. Regulation of activated T cell survival in rheumatic autoimmune diseases. Nat. Rev. Rheumatol. 18, 232–244 (2022).
Sumida, T. S., Cheru, N. T. & Hafler, D. A. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-024-00994-x (2024).
Kolios, A. G. A., Tsokos, G. C. & Klatzmann, D. Interleukin-2 and regulatory T cells in rheumatic diseases. Nat. Rev. Rheumatol. 17, 749–766 (2021).
Bailey-Bucktrout, S. L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Li, J. et al. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ. Res. 118, 1540–1552 (2016).
Butcher, M. J. et al. Atherosclerosis-driven treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ Th1/Tregs. Circ. Res. 119, 1190–1203 (2016).
Kimura, T. et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation 138, 1130–1143 (2018).
Spee-Mayer et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 78, 209–217 (2019).
He, J. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 79, 141–149 (2020).
Akbarzadeh, R., Riemekasten, G. & Humrich, J. Y. Low-dose interleukin-2 therapy: a promising targeted therapeutic approach for systemic lupus erythematosus. Curr. Opin. Rheumatol. 35, 98–106 (2023).
Sriranjan, R. et al. Low-dose interleukin 2 for the reduction of vascular inflammation in acute coronary syndromes (IVORY): protocol and study rationale for a randomised, double-blind, placebo-controlled, phase II clinical trial. BMJ Open 12, e062602 (2022).
Koshy, M., Berger, D. & Crow, M. K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Invest. 98, 826–837 (1996).
Liossis, S. N., Ding, X. Z., Dennis, G. J. & Tsokos, G. C. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J. Clin. Invest. 101, 1448–1457 (1998).
Enyedy, E. J. et al. Fcϵ receptor type I γ chain replaces the deficient T cell receptor ζ chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 44, 1114–1121 (2001).
Wagner, U. G., Koetz, K., Weyand, C. M. & Goronzy, J. J. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 95, 14447–14452 (1998).
Weyand, C. M. & Goronzy, J. J. The immunology of rheumatoid arthritis. Nat. Immunol. 22, 10–18 (2021).
Weng, N., Akbar, A. N. & Goronzy, J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Dumitriu, I. E. et al. High levels of costimulatory receptors OX40 and 4-1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ. Res. 110, 857–869 (2012).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Téo, F. H. et al. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell. Immunol. 281, 11–19 (2013).
Okba, A. M. et al. Expanded peripheral CD4+CD28null T cells and its association with atherosclerotic changes in patients with end stage renal disease on hemodialysis. Hum. Immunol. 80, 748–754 (2019).
Gerli, R. et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109, 2744–2748 (2004).
Jiang, Q. et al. Role of Th22 cells in the pathogenesis of autoimmune diseases. Front. Immunol. 12, 688066 (2021).
Schnell, A., Littman, D. R. & Kuchroo, V. K. TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 24, 19–29 (2023).
Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62, 234–244 (2010).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, 601–610 (2017).
Clement, M. et al. Control of the T follicular helper–germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 560–570 (2015).
Bocharnikov, A. V. et al. PD-1hiCXCR5− T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 4, e130062 (2019).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42, 1100–1115 (2015).
Srikakulapu, P. et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-cell responses in aged ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 36, 1174–1185 (2016).
Paulsson, G., Zhou, X., Törnquist, E. & Hansson, G. K. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20, 10–17 (2000).
Lin, Z. et al. Deep sequencing of the T cell receptor β repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget 8, 99312–99322 (2017).
Sage, A. P., Tsiantoulas, D., Binder, C. J. & Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 16, 180–196 (2019).
Chaturvedi, A., Dorward, D. & Pierce, S. K. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity 28, 799–809 (2008).
Groom, J. R. et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 204, 1959–1971 (2007).
Rubtsov, A. V. et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 118, 1305–1315 (2011).
Naradikian, M. S. et al. Cutting edge: IL-4, IL-21, and IFN-γ interact to govern T-bet and CD11c expression in TLR-activated B cells. J. Immunol. 197, 1023–1028 (2016).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Guerrier, T., Youinou, P., Pers, J.-O. & Jamin, C. TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J. Autoimmun. 39, 173–179 (2012).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Dörner, T., Jacobi, A. M., Lee, J. & Lipsky, P. E. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. Immunol. Methods 363, 187–197 (2011).
Meeuwsen, J. A. L. et al. High levels of (un)switched memory B cells are associated with better outcome in patients with advanced atherosclerotic disease. J. Am. Heart Assoc. 6, e005747 (2017).
She, Z. et al. The role of B1 cells in systemic lupus erythematosus. Front. Immunol. 13, 814857 (2022).
Mantovani, L., Wilder, R. L. & Casali, P. Human rheumatoid B-1a (CD5+ B) cells make somatically hypermutated high affinity IgM rheumatoid factors. J. Immunol. 151, 473–488 (1993).
Peng, S. L., Szabo, S. J. & Glimcher, L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl Acad. Sci. USA 99, 5545–5550 (2002).
Rubtsova, K. et al. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Invest. 127, 1392–1404 (2017).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102, 1596–1601 (2005).
Li, Z.-Y., Cai, M.-L., Qin, Y. & Chen, Z. Age/autoimmunity-associated B cells in inflammatory arthritis: an emerging therapeutic target. Front. Immunol. 14, 1103307 (2023).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Qin, Y. et al. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-α-mediated ERK1/2 and JAK-STAT1 pathways. Ann. Rheum. Dis. 81, 1504–1514 (2022).
Smit, V. et al. Single-cell profiling reveals age-associated immunity in atherosclerosis. Cardiovasc. Res. 119, 2508–2521 (2023).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 207, 1579–1587 (2010).
Kyaw, T. et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 185, 4410–4419 (2010).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT05211401 (2023).
Giordano, D. et al. B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease. Front. Immunol. 14, 1050528 (2023).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Hamilton, J. A., Hsu, H.-C. & Mountz, J. D. Autoreactive B cells in SLE, villains or innocent bystanders? Immunol. Rev. 292, 120–138 (2019).
Zhang, J. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166, 6–10 (2001).
Salazar-Camarena, D. C. et al. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 25, 582–592 (2016).
Stohl, W. et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 48, 3475–3486 (2003).
Landolt-Marticorena, C. et al. Increased expression of B cell activation factor supports the abnormal expansion of transitional B cells in systemic lupus erythematosus. J. Rheumatol. 38, 642–651 (2011).
Sage, A. P. et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice – brief report. Arterioscler. Thromb. Vasc. Biol. 32, 1573–1576 (2012).
Kyaw, T. et al. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PloS ONE 7, e29371 (2012).
Jackson, S. W. et al. Cutting edge: BAFF overexpression reduces atherosclerosis via TACI-dependent B cell activation. J. Immunol. 197, 4529–4534 (2016).
Tsiantoulas, D. et al. B cell-activating factor neutralization aggravates atherosclerosis. Circulation 138, 2263–2273 (2018).
Saidoune, F. et al. Effects of BAFF neutralization on atherosclerosis associated with systemic lupus erythematosus. Arthritis Rheumatol. 73, 255–264 (2021).
Mauri, C. & Blair, P. A. Regulatory B cells in autoimmunity: developments and controversies. Nat. Rev. Rheumatol. 6, 636–643 (2010).
Sakkas, L. I., Daoussis, D., Mavropoulos, A., Liossis, S.-N. & Bogdanos, D. P. Regulatory B cells: new players in inflammatory and autoimmune rheumatic diseases. Semin. Arthritis Rheum. 48, 1133–1141 (2019).
Meng, X. et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat. Commun. 9, 251 (2018).
Gao, N. et al. Impaired suppressive capacity of activation-induced regulatory B cells in systemic lupus erythematosus. Arthritis Rheumatol. 66, 2849–2861 (2014).
Smith, K. G. C. & Clatworthy, M. R. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 10, 328–343 (2010).
Bagchi-Chakraborty, J. et al. B cell Fcγ receptor IIb modulates atherosclerosis in male and female mice by controlling adaptive germinal center and innate B-1-cell responses. Arterioscler. Thromb. Vasc. Biol. 39, 1379–1389 (2019).
Pisetsky, D. S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 19, 509–524 (2023).
Lewis, M. J. et al. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor–deficient mice. Circulation 120, 417–426 (2009).
Tsiantoulas, D. et al. Increased plasma IgE accelerate atherosclerosis in secreted IgM deficiency. Circ. Res. 120, 78–84 (2017).
Ebrahimian, T. et al. B cell-specific knockout of AID protects against atherosclerosis. Sci. Rep. 13, 8723 (2023).
Centa, M. et al. Germinal center-derived antibodies promote atherosclerosis plaque size and stability. Circulation 139, 2466–2482 (2019).
Tay, C. et al. Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 38, e71–e84 (2018).
Mackey, R. H. et al. Rheumatoid arthritis, anti-cyclic citrullinated peptide positivity, and cardiovascular disease risk in the Women’s Health Initiative. Arthritis Rheumatol. 67, 2311–2322 (2015).
Hörkkö, S. et al. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. Recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J. Clin. Invest. 98, 815–825 (1996).
Deroissart, J. & Binder, C. J. Mapping the functions of IgM antibodies in atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 20, 433–434 (2023).
Grönwall, C. et al. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin. Immunol. 142, 390–398 (2012).
Thiagarajan, D. et al. IgM antibodies against malondialdehyde and phosphorylcholine in different systemic rheumatic diseases. Sci. Rep. 10, 11010 (2020).
Anania, C. et al. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res. Ther. 12, R214 (2010).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740 (2000).
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 (2003).
Chen, Y. et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J. Immunol. 183, 1346–1359 (2009).
Faria-Neto, J. R. et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 189, 83–90 (2006).
Urowitz, M. B., Ibañez, D., Su, J. & Gladman, D. D. Modified Framingham Risk Factor Score for systemic lupus erythematosus. J. Rheumatol. 43, 875–879 (2016).
Hippisley-Cox, J., Coupland, C. & Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 357, j2099 (2017).
Petri, M. A., Barr, E. & Magder, L. S. Development of a systemic lupus erythematosus cardiovascular risk equation. Lupus Sci. Med. 6, e000346 (2019).
McMahon, M. et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 66, 130–139 (2014).
Skaggs, B. J. et al. A panel of biomarkers associates with increased risk for cardiovascular events in women with systemic lupus erythematosus. ACR Open Rheumatol. 3, 209–220 (2021).
Sivakumaran, J. et al. Assessment of cardiovascular risk tools as predictors of cardiovascular disease events in systemic lupus erythematosus. Lupus Sci. Med. 8, e000448 (2021).
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2017).
Drosos, G. C. et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 81, 768–779 (2022).
Buckley, L. F. & Libby, P. Colchicine’s role in cardiovascular disease management. Arterioscler. Thromb. Vasc. Biol. 44, 1031–1041 (2024).
Wade, N. S., Stevenson, B. G., Dunlap, D. S. & Major, A. S. The lupus susceptibility locus Sle3 is not sufficient to accelerate atherosclerosis in lupus-susceptible low density lipoprotein receptor-deficient mice. Lupus 19, 34–42 (2010).
Asquith, D. L. et al. Apolipoprotein E-deficient mice are resistant to the development of collagen-induced arthritis. Arthritis Rheum. 62, 472–477 (2010).
Blackler, G. et al. The effect of HLA-DRB1*04:01 on a mouse model of atherosclerosis. J. Transl. Autoimmun. 7, 100203 (2023).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Nidorf, S. M., Eikelboom, J. W., Budgeon, C. A. & Thompson, P. L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 61, 404–410 (2013).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT03048825 (2024).
Kelly, P. et al. Long-term colchicine for the prevention of vascular recurrent events in non-cardioembolic stroke (CONVINCE): a randomised controlled trial. Lancet https://doi.org/10.1016/S0140-6736(24)00968-1 (2024).
Morton, A. C. et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Heart J. 36, 377–384 (2015).
Abbate, A. et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot Study). Am. J. Cardiol. 105, 1371–1377.e1 (2010).
Abbate, A. et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University–Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 111, 1394–1400 (2013).
Abbate, A. et al. Interleukin‐1 blockade inhibits the acute inflammatory response in patients with ST‐segment-elevation myocardial infarction. J. Am. Heart Assoc. 9, e014941 (2020).
Kleveland, O. et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 37, 2406–2413 (2016).
Kleveland, O. et al. Interleukin-6 receptor inhibition with tocilizumab induces a selective and substantial increase in plasma IP-10 and MIP-1β in non-ST-elevation myocardial infarction. Int. J. Cardiol. 271, 1–7 (2018).
Carroll, M. B., Haller, C. & Smith, C. Short-term application of tocilizumab during myocardial infarction (STAT-MI). Rheumatol. Int. 38, 59–66 (2018).
Broch, K. et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855 (2021).
Kunkel, J. B. et al. Low-dose dobutamine infusion and single-dose tocilizumab in acute myocardial infarction patients with high risk of cardiogenic shock development – rationale and design of the DOBERMANN trial [abstract zuad036.131]. Eur. Heart J. Acute Cardiovasc. Care 12 (Suppl. 1), i193–i194 (2023).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT05350592 (2024).
Ridker, P. M. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc. Res. 117, e138–e140 (2021).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT06118281 (2024).
Zhao, T. X. et al. Rituximab in patients with acute ST-elevation myocardial infarction: an experimental medicine safety study. Cardiovasc. Res. 118, 872–882 (2022).
Zhao, T. X. et al. Regulatory T-cell response to low-dose interleukin-2 in ischemic heart disease. NEJM Evid. 1, EVIDoa2100009 (2022).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/ct2/show/NCT04241601 (2024).
Borén, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 41, 2313–2330 (2020).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Hung, T. et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 350, 455–459 (2015).
Kudo, T. et al. Regulation of NETosis and inflammation by cyclophilin D in myeloperoxidase-positive antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 75, 71–83 (2023).
Müller-Calleja, N. et al. Lipid presentation by the protein C receptor links coagulation with autoimmunity. Science 371, eabc0956 (2021).
Schreiber, K. et al. Antiphospholipid syndrome. Nat. Rev. Dis. Prim. 4, 17103 (2018).
Pagano, S. et al. Anti-apolipoprotein A-1 IgG in patients with myocardial infarction promotes inflammation through TLR2/CD14 complex. J. Intern. Med. 272, 344–357 (2012).
Kitching, A. R. et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 6, 71 (2020).
van Delft, M. A. M. & Huizinga, T. W. J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 110, 102392 (2020).
Vander Cruyssen, B. et al. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun. Rev. 4, 468–474 (2005).
Anquetil, F., Clavel, C., Offer, G., Serre, G. & Sebbag, M. IgM and IgA rheumatoid factors purified from rheumatoid arthritis sera boost the Fc receptor- and complement-dependent effector functions of the disease-specific anti-citrullinated protein autoantibodies. J. Immunol. 194, 3664–3674 (2015).
Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202 (2015).
Acknowledgements
C.J.B. was supported by grants from the Vienna Science and Technology Fund (LS18-090), by the Leducq Foundation (Transatlantic Network of Excellence; TNE-20CVD03) and the EU Horizon Europe (TillT; 101080897).
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Jan Nilsson, Michael Nurmohamed and Marten Trendelenburg for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Clonal haematopoiesis of indeterminate potential
-
(CHIP). A common, age-related condition in which somatic mutations in some genes in haematopoietic progenitor cells result in the clonal expansion of leukocytes. The presence of CHIP is associated with a significantly increased risk of cardiovascular disease.
- Endothelial-to-mesenchymal transition
-
A process in which endothelial cells change their molecular and cellular phenotype to that of mesenchymal cells (such as myofibroblasts and smooth muscle cells). Endothelial-to-mesenchymal transition has been implicated in the development of atherosclerosis.
- Lipoprotein(a)
-
An LDL particle that contains apolipoprotein(a). Lipoprotein(a) is one of the strongest genetically determined risk factors for atherosclerotic cardiovascular disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Porsch, F., Binder, C.J. Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol (2024). https://doi.org/10.1038/s41569-024-01045-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-024-01045-7
This article is cited by
-
Atheroimmunology: keeping the immune system in atherosclerosis in check
Nature Reviews Cardiology (2024)