Abstract
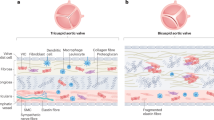
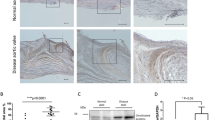
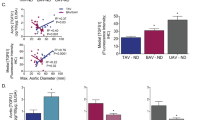
Calcific aortic valve disease (CAVD) and stenosis have a complex pathogenesis, and no therapies are available that can halt or slow their progression. Several studies have shown the presence of apolipoprotein-related amyloid deposits in close proximity to calcified areas in diseased aortic valves. In this Perspective, we explore a possible relationship between amyloid deposits, calcification and the development of aortic valve stenosis. These amyloid deposits might contribute to the amplification of the inflammatory cycle in the aortic valve, including extracellular matrix remodelling and myofibroblast and osteoblast-like cell proliferation. Further investigation in this area is needed to characterize the amyloid deposits associated with CAVD, which could allow the use of antisense oligonucleotides and/or isotype gene therapies for the prevention and/or treatment of CAVD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
17 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41569-023-00848-4
References
Lindman, B. R. et al. Calcific aortic stenosis. Nat. Rev. Dis. Prim. 2, 16006 (2016).
Aikawa, E. & Hutcheson, J. D. The developmental origin of calcific aortic stenosis. N. Engl. J. Med. 386, 1372–1374 (2022).
Rapp, A. H., Hillis, L. D., Lange, R. A. & Cigarroa, J. E. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris [abstract A1217]. Am. J. Cardiol. 87, 1216–1217 (2001).
Thanassoulis, G. et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368, 503–512 (2013).
Yan, A. T. et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART Aortic Stenosis study. J. Am. Coll. Cardiol. 69, 1523–1532 (2017).
Chan, K. L. et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121, 306–314 (2010).
Cowell, S. J. et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 352, 2389–2397 (2005).
Rossebo, A. B. et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359, 1343–1356 (2008).
Goffin, Y. Microscopic amyloid deposits in the heart valves: a common local complication of chronic damage and scarring. J. Clin. Pathol. 33, 262–268 (1980).
Buxbaum, J. N. et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid https://doi.org/10.1080/13506129.2022.2147636 (2022).
Sawaya, M. R. et al. The expanding amyloid family: structure, stability, function, and pathogenesis. Cell 184, 4857–4873 (2021).
Wisniewski, T. & Drummond, E. ApoE-amyloid interaction: therapeutic targets. Neurobiol. Dis. 138, 104784 (2020).
Lewkowicz, E., Jayaraman, S. & Gursky, O. Protein amyloid cofactors: charged side-chain arrays meet their match? Trends Biochem. Sci. 46, 626–629 (2021).
Tao, Y. et al. Heparin induces α-synuclein to form new fibril polymorphs with attenuated neuropathology. Nat. Commun. 13, 4226 (2022).
Kittleson, M. M. et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation 142, E7–E22 (2020).
Obici, L. et al. Structure, function and amyloidogenic propensity of apolipoprotein A-1. Amyloid 13, 191–205 (2006).
Rocken, C. et al. Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler. Thromb. Vasc. Biol. 26, 676–677 (2006).
Wong, Y. Q., Binger, K. J., Howlett, G. J. & Griffin, M. D. Methionine oxidation induces amyloid fibril formation by full-length apolipoprotein A-I. Proc. Natl Acad. Sci. USA 107, 1977–1982 (2010).
Zhao, L., Buxbaum, J. N. & Reixach, N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 52, 1913–1926 (2013).
Iwata, T. et al. Amyloid deposits in heart valves. Acta Pathol. Jpn 32, 23–29 (1982).
Cooper, J. H. Localized dystrophic amyloidosis of heart valves. Hum. Pathol. 14, 649–653 (1983).
Kristen, A. V. et al. High prevalence of amyloid in 150 surgically removed heart valves: a comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc. Pathol. 19, 228–235 (2010).
Audet, A. et al. Amyloid substance within stenotic aortic valves promotes mineralization. Histopathology 61, 610–619 (2012).
Ladefoged, C. & Rohr, N. Amyloid deposits in aortic and mitral valves: a clinicopathological investigation of material from 100 consecutive heart valve operations. Virchows Arch. A Pathol. Anat. Histopathol. 404, 301–312 (1984).
Das, M. & Gursky, O. Amyloid-forming properties of human apolipoproteins: sequence analyses and structural insights. Adv. Exp. Med. Biol. 855, 175–211 (2015).
Tintut, Y., Hsu, J. J. & Demer, L. L. Lipoproteins in cardiovascular calcification: potential targets and challenges. Front. Cardiovasc. Med. 5, 172 (2018).
Mamarelis, I. et al. FT-IR spectroscopic study of amyloid protein formation and aortic valve calcification. Hell. J. Cardiol. 58, 148–150 (2017).
Schlotter, F. et al. ApoC-III is a novel inducer of calcification in human aortic valves. J. Biol. Chem. 296, 100193 (2021).
Zuckerman, S. H., Evans, G. F. & Oneal, L. Cytokine regulation of macrophage apo E secretion: opposing effects of GM-CSF and TGF-β. Atherosclerosis 96, 203–214 (1992).
Novaro, G. M. et al. Association between apolipoprotein E alleles and calcific valvular heart disease. Circulation 108, 1804–1808 (2003).
Lommi, J. I. et al. High-density lipoproteins (HDL) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis 219, 538–544 (2011).
Schlotter, F. et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 138, 377–393 (2018).
Capoulade, R. et al. ApoCIII-Lp(a) complexes in conjunction with L(a)-OxPL predict rapid progression of aortic stenosis. Heart 106, 738–745 (2020).
Kaiser, Y. et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur. Heart J. 43, 3960–3967 (2022).
Capoulade, R. et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66, 1236–1246 (2015).
Zheng, K. H. et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J. Am. Coll. Cardiol. 73, 2150–2162 (2019).
Bhatia, H. S. et al. Trends in testing and prevalence of elevated Lp(a) among patients with aortic valve stenosis. Atherosclerosis 349, 144–150 (2022).
Kamstrup, P. R. et al. Oxidized phospholipids and risk of calcific aortic valve disease: the Copenhagen General Population Study. Arterioscler. Thromb. Vasc. Biol. 37, 1570–1578 (2017).
Torzewski, M. et al. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. JACC Basic Transl Sci. 2, 229–240 (2017).
Heuschkel, M. A. et al. Integrative multi-omics analysis in calcific aortic valve disease reveals a link to the formation of amyloid-like deposits. Cells 9, 2164 (2020).
Xiong, M. et al. ApoE immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Sci. Transl Med. 13, eabd7522 (2021).
Jayaraman, S., Sanchez-Quesada, J. L. & Gursky, O. Triglyceride increase in the core of high-density lipoproteins augments apolipoprotein dissociation from the surface: potential implications for treatment of apolipoprotein deposition diseases. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 200–210 (2017).
Medeiros, L. A. et al. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J. Biol. Chem. 279, 10643–10648 (2004).
Luciunaite, A. et al. Soluble abeta oligomers and protofibrils induce NLRP3 inflammasome activation in microglia. J. Neurochem. 155, 650–661 (2020).
Martin-Rojas, T. et al. iTRAQ proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci. Rep. 5, 17290 (2015).
Meng, X. Z. et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am. J. Physiol. Cell Physiol. 294, C29–C35 (2008).
Cottignoli, V., Cavarretta, E., Salvador, L., Valfre, C. & Maras, A. Morphological and chemical study of pathological deposits in human aortic and mitral valve stenosis: a biomineralogical contribution. Pathol. Res. Int. 2015, 342984 (2015).
Ternacle, J. et al. Aortic stenosis and cardiac amyloidosis. J. Am. Coll. Cardiol. 74, 2638–2651 (2019).
Mamarelis, I. et al. The role of oxidative stress on amyloid-like protein formation and aortic valve calcification. Eur. Heart J. 37, 313 (2016).
Dittfeld, C. et al. Molecular spectroscopic imaging offers a systematic assessment of pathological aortic valve and prosthesis tissue in biomineralization. Crystals 10, 763 (2020).
Stats, M. A. & Stone, J. R. Varying levels of small microcalcifications and macrophages in attr and al cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 25, 413–417 (2016).
Simard, L. et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ. Res. 120, 681–691 (2017).
Caponetti, A. G. et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from THAOS. JACC Heart Fail. 9, 736–746 (2021).
Jenkins, M. C. & Potter, M. Calcified pseudotumoural mediastinal amyloidosis. Thorax 46, 686–687 (1991).
Westermark, P., Mucchiano, G., Marthin, T., Johnson, K. H. & Sletten, K. Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am. J. Pathol. 147, 1186–1192 (1995).
Li, X. A., Hatanaka, K., Ishibashi-Ueda, H., Yutani, C. & Yamamoto, A. Characterization of serum amyloid P component from human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 15, 252–257 (1995).
Howlett, G. J. & Moore, K. J. Untangling the role of amyloid in atherosclerosis. Curr. Opin. Lipidol. 17, 541–547 (2006).
Hellberg, S. et al. Amyloid-targeting PET tracer [18F]flutemetamol accumulates in atherosclerotic plaques. Molecules 24, 1072 (2019).
Abdelbaky, A. et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ. Cardiovasc. Imaging 6, 747–754 (2013).
Akkineni, S. et al. Amyloid-like amelogenin nanoribbons template mineralization via a low-energy interface of ion binding sites. Proc. Natl Acad. Sci. USA 119, e2106965119 (2022).
Bai, Y. et al. Protein nanoribbons template enamel mineralization. Proc. Natl Acad. Sci. USA 117, 19201–19208 (2020).
Ajili, W. et al. Inorganic phosphate in growing calcium carbonate abalone shell suggests a shared mineral ancestral precursor. Nat. Commun. 13, 1496 (2022).
Iadanza, M. G. et al. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 19, 755–773 (2018).
Yang, Y. et al. Cryo-EM structures of amyloid-β 42 filaments from human brains. Science 375, 167–172 (2022).
Poulsen, E. T., Pedersen, K. W., Marzeda, A. M. & Enghild, J. J. Serum amyloid P component (SAP) interactome in human plasma containing physiological calcium levels. Biochemistry 56, 896–902 (2017).
Richards, D. B. et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 373, 1106–1114 (2015).
Moura, L. M. et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J. Am. Coll. Cardiol. 49, 554–561 (2007).
Tsimikas, S. et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 382, 244–255 (2020).
Acknowledgements
E. Aikawa has received support from the NIH (grant numbers R01 HL136431, R01 HL141917 and R01 HL147095). S.G. has received support from the National Institute on Aging – Designated Alzheimer’s Disease Research Center (P50 AG005138 and P30 AG066514). O.G. has received support from the NIH (grants R01 GM067260 and R01 GM135158).
Author information
Authors and Affiliations
Contributions
K.S. and N.N. researched data for the article. K.S., N.N., E. Aikawa, E. Arbustini, P.P., G.M., R.S.R., E. Argulian and J.N. discussed the content of the article. K.S., N.N., S.G., O.G. and J.N. wrote the manuscript. All the authors reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
P.P. has received grant funding from Cardiac Phoenix, Edwards Lifesciences, Medtronic and Pi-Cardia for echocardiography core laboratory analyses and research studies in the field of transcatheter valve therapies, for which he received no personal compensation; he has also received lecture fees from Edwards Lifesciences and Medtronic. R.S.R. has received research grants to his institution from Amgen, Arrowhead, NIH, Novartis and Regeneron, is a member of the advisory board of Amgen, Novartis, Regeneron and 89 Bio, has received honoraria for non-promotional speaking from Kowa, has stock holdings with MediMergent, and receives royalties from Wolters Kluwer (UpToDate). S.T. is a co-inventor who receives royalties from patents owned by University of California, San Diego on oxidation-specific antibodies and biomarkers related to oxidized lipoproteins, and is a co-founder who has equity interests in Kleanthi Diagnostics and in Oxitope and affiliates. Although these relationships have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Kleanthi and Oxitope, the research findings included in this particular publication do not necessarily relate to the interests of Kleanthi and Oxitope. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with conflict of interest policies. S.G. serves as a consultant for Altpep, Cognition Therapeutics, and Ritrova Therapeutics and is a founder of Recuerdo Pharmaceuticals (inactive), has served as a consultant in the past for Diagenic, has received research support in the past from Avid, Baxter, Pfizer and Warner-Lambert, and also receives compensation for chart review in connection with medical litigation in the area of cognitive function. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Matthew Budoff, Romain Capoulade and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sud, K., Narula, N., Aikawa, E. et al. The contribution of amyloid deposition in the aortic valve to calcification and aortic stenosis. Nat Rev Cardiol 20, 418–428 (2023). https://doi.org/10.1038/s41569-022-00818-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00818-2
This article is cited by
-
Osteopontin stabilization and collagen containment slows amorphous calcium phosphate transformation during human aortic valve leaflet calcification
Scientific Reports (2024)
-
Valvular heart disease in patients with cardiac amyloidosis
Heart Failure Reviews (2023)