Abstract
Although acute coronary syndromes (ACS) remain one of the leading causes of death, the clinical presentation has changed over the past three decades with a decline in the incidence of ST-segment elevation myocardial infarction (STEMI) and an increase in non-STEMI. This epidemiological shift is at least partially explained by changes in plaque biology as a result of the widespread use of statins. Historically, atherosclerotic plaque rupture of the fibrous cap was thought to be the main culprit in ACS. However, plaque erosion with an intact fibrous cap is now responsible for about one third of ACS and up to two thirds of non-STEMI. Two major research approaches have enabled a better understanding of plaque erosion. First, advanced intravascular imaging has provided opportunities for an ‘optical biopsy’ and extensive phenotyping of coronary plaques in living patients. Second, basic science experiments have shed light on the unique molecular characteristics of plaque erosion. At present, patients with ACS are still uniformly treated with coronary stents irrespective of the underlying pathobiology. However, pilot studies indicate that patients with plaque erosion might be treated conservatively without coronary stenting. In this Review, we discuss the patient phenotype and the molecular characteristics in atherosclerotic plaque erosion and provide our vision for a potential major shift in the management of patients with plaque erosion.
Key points
-
Plaque erosion rather than plaque rupture has become the predominant mechanism of non-ST-segment elevation myocardial infarction as a result of better control of cardiovascular risk factors since the introduction of statins.
-
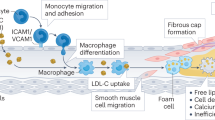
Plaque erosion starts with changes in endothelial shear stress gradients that activate Toll-like receptor 2 in endothelial cells, resulting in loss of basement membrane integrity and endothelial cell desquamation, with subsequent formation of neutrophil extracellular traps and thrombosis.
-
Optical coherence tomography has enabled the in vivo diagnosis of plaque erosion, providing a better understanding of the characteristics and vascular biology of plaque erosion in patients with acute coronary syndromes.
-
Plaque erosion is characterized by preserved vascular integrity, a larger vessel lumen than in plaque rupture and the presence of a platelet-rich thrombus and, overall, is associated with a better risk profile and outcomes than plaque rupture.
-
Multiple clinical, angiographic and laboratory predictors of plaque erosion can help identify patients with plaque erosion, but more specific point-of-care biomarkers and non-invasive imaging techniques are needed.
-
Early clinical studies suggest that patients with plaque erosion might be managed without percutaneous coronary intervention, ushering in a new era of precision medicine in the management of patients with acute coronary syndromes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Antman, E. M. & Loscalzo, J. Precision medicine in cardiology. Nat. Rev. Cardiol. 13, 591–602 (2016).
Virani, S. S. et al. Heart disease and stroke statistics–2020 update: a report from the American Heart Association. Circulation 141, e139–e596 (2020).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130, 2354–2394 (2014).
Antman, E.M. et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 110, e82–e292 (2004).
Prati, F. et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 31, 401–415 (2010).
Crea, F. & Libby, P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 136, 1155–1166 (2017).
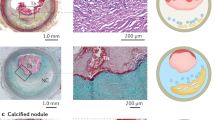
Jia, H. et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 62, 1748–1758 (2013).
Higuma, T. et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 8, 1166–1176 (2015).
Saia, F. et al. Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI. JACC Cardiovasc. Imaging 8, 566–575 (2015).
Niccoli, G. et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 36, 1377–1384 (2015).
Yonetsu, T. et al. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int. J. Cardiol. 203, 766–774 (2016).
Kajander, O. A. et al. Culprit plaque morphology in STEMI – an optical coherence tomography study: insights from the TOTAL-OCT substudy. EuroIntervention 12, 716–723 (2016).
Kwon, J. E. et al. Multimodality intravascular imaging assessment of plaque erosion versus plaque rupture in patients with acute coronary syndrome. Korean Circ. J. 46, 499–506 (2016).
Libby, P., Pasterkamp, G., Crea, F. & Jang, I.-K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 124, 150–160 (2019).
Jia, H. et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 38, 792–800 (2016).
van der Wal, A. C., Becker, A. E., van der Loos, C. M. & Das, P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89, 36–44 (1994).
Farb, A. et al. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation 93, 1354–1363 (1996).
Burke, A. P. et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 336, 1276–1282 (1997).
Arbustini, E. et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 82, 269–272 (1999).
Muller, J. E., Tofler, G. H. & Stone, P. H. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79, 733–743 (1989).
Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 50, 127–134 (1983).
Davies, M. J. & Thomas, A. C. Plaque fissuring–the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 53, 363–373 (1985).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Burke, A. P. et al. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation 97, 2110–2116 (1998).
Burke, A. P. et al. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation 105, 419–424 (2002).
Durand, E. et al. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 109, 2503–2506 (2004).
Henriques de Gouveia, R. Sudden unexpected death in young adults. Discrepancies between initiation of acute plaque complications and the onset of acute coronary death. Eur. Heart J. 23, 1433–1440 (2002).
Hu, S. et al. Management and outcome of patients with acute coronary syndrome caused by plaque rupture versus plaque erosion: an intravascular optical coherence tomography study. J. Am. Heart Assoc. 6, e00473 (2017).
Kolodgie, F. D. et al. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler. Thromb. Vasc. Biol. 22, 1642–1648 (2002).
Kramer, M. C. A. et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J. Am. Coll. Cardiol. 55, 122–132 (2010).
Sato, Y. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart 91, 526–530 (2005).
Schwartz, R. S. et al. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death. J. Am. Coll. Cardiol. 54, 2167–2173 (2009).
Tavora, F. et al. Sudden coronary death caused by pathologic intimal thickening without atheromatous plaque formation. Cardiovasc. Pathol. 20, 51–57 (2011).
Stone, G. W. et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235 (2011).
Puri, R., Worthley, M. I. & Nicholls, S. J. Intravascular imaging of vulnerable coronary plaque: current and future concepts. Nat. Rev. Cardiol. 8, 131–139 (2011).
Kubo, T. et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50, 933–939 (2007).
Hong, S. et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA 314, 2155–2163 (2015).
Zhang, J. et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J. Am. Coll. Cardiol. 72, 3126–3137 (2018).
Pasterkamp, G., den Ruijter, H. M. & Libby, P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat. Rev. Cardiol. 14, 21–29 (2017).
Hayashi, T. et al. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am. Heart J. 149, 284–290 (2005).
Sun, R. et al. Pre-infarction angina and culprit lesion morphologies in patients with a first ST-segment elevation acute myocardial infarction: insights from in vivo optical coherence tomography. EuroIntervention 14, 1768–1775 (2019).
Prati, F. et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc. Imaging 6, 283–287 (2013).
Yahagi, K., Davis, H. R., Arbustini, E. & Virmani, R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis 239, 260–267 (2015).
Yamamoto, E. et al. Clinical and laboratory predictors for plaque erosion in patients with acute coronary syndromes. J. Am. Heart Assoc. 8, e012322 (2019).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Niccoli, G. et al. Morphological-biohumoral correlations in acute coronary syndromes: pathogenetic implications. Int. J. Cardiol. 171, 463–466 (2014).
Dai, J. et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur. Heart J. 39, 2077–2085 (2018).
Papaioannou, T. G. & Stefanadis, C. Vascular wall shear stress: basic principles and methods. Hellenic J. Cardiol. 46, 9–15 (2005).
Ferrante, G. et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation 122, 2505–2513 (2010).
Pedicino, D. et al. Alterations of hyaluronan metabolism in acute coronary syndrome: implications for plaque erosion. J. Am. Coll. Cardiol. 72, 1490–1503 (2018).
Chandran, S. et al. Inflammatory differences in plaque erosion and rupture in patients with ST-segment elevation myocardial infarction. J. Am. Heart Assoc. 6, e005868 (2017).
Tian, J. et al. Morphologic characteristics of eroded coronary plaques: a combined angiographic, optical coherence tomography, and intravascular ultrasound study. Int. J. Cardiol. 176, e137–e139 (2014).
Vergallo, R. et al. Dual quantitative coronary angiography accurately quantifies intracoronary thrombotic burden in patients with acute coronary syndrome: comparison with optical coherence tomography imaging. Int. J. Cardiol. 292, 25–31 (2019).
Kim, H. O. et al. Angiographic features of patients with coronary plaque erosion. Int. J. Cardiol. 288, 12–16 (2019).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 51, 606 (1983).
Sianos, G. et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1, 219–227 (2005).
Neeland, I. J. et al. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am. Heart J. 164, 547–552.e1 (2012).
Ryan, T. J. Guidelines for percutaneous transluminal coronary angioplasty: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). J. Am. Coll. Cardiol. 12, 529–545 (1988).
Gijsen, F. J. et al. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J. Biomech. 40, 2349–2357 (2007).
Araki, M. et al. Spatial distribution of vulnerable plaques: comprehensive in vivo coronary plaque mapping. JACC Cardiovasc. Imaging 13, 1989–1999 (2020).
Thondapu, V. et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvaa251 (2020).
Vergallo, R. et al. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ. Cardiovasc. Imaging 7, 905–911 (2014).
Vergallo, R. et al. Coronary plaque erosion developing in an area of high endothelial shear stress: insights from serial optical coherence tomography imaging. Coron. Artery Dis. 30, 74–75 (2019).
Yamamoto, E. et al. Endothelial shear stress and plaque erosion: a computational fluid dynamics and optical coherence tomography study. JACC Cardiovasc. Imaging 12, 374–375 (2019).
Matsuura, Y., Kanter, J. E. & Bornfeldt, K. E. Highlighting residual atherosclerotic cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 39, e1–e9 (2019).
Mullick, A. E., Tobias, P. S. & Curtiss, L. K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115, 3149–3156 (2005).
Mullick, A. E. et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 205, 373–383 (2008).
Rajavashisth, T. B. et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J. Biol. Chem. 274, 11924–11929 (1999).
Franck, G. et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ. Res. 121, 31–42 (2017).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Scheibner, K. A. et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 (2006).
Taylor, K. R. et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 279, 17079–17084 (2004).
Bertheloot, D. & Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell. Mol. Immunol. 14, 43–64 (2017).
Laman, J. D., Schoneveld, A. H., Moll, F. L., van Meurs, M. & Pasterkamp, G. Significance of peptidoglycan, a proinflammatory bacterial antigen in atherosclerotic arteries and its association with vulnerable plaques. Am. J. Cardiol. 90, 119–123 (2002).
Nijhuis, M. M. O. et al. Peptidoglycan increases firm adhesion of monocytes under flow conditions and primes monocyte chemotaxis. J. Vasc. Res. 44, 214–222 (2007).
Wright, H. L., Moots, R. J., Bucknall, R. C. & Edwards, S. W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49, 1618–1631 (2010).
Borregaard, N., Sørensen, O. E. & Theilgaard-Mönch, K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28, 340–345 (2007).
Naegelen, I. et al. Regulation of neutrophil degranulation and cytokine secretion: a novel model approach based on linear fitting. J. Immunol. Res. 2015, 817038 (2015).
Folco, E. J. et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 38, 1901–1912 (2018).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA 107, 15880–15885 (2010).
Martinod, K. & Wagner, D. D. Thrombosis: tangled up in NETs. Blood 123, 2768–2776 (2014).
Chow, O. A. et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8, 445–454 (2010).
Soehnlein, O., Bazioti, V. & Westerterp, M. A Pad 4 plaque erosion. Circ. Res. 123, 6–8 (2018).
Franck, G. et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ. Res. 123, 33–42 (2018).
Cooley, B. C. et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 6, 227ra34 (2014).
Wesseling, M., Sakkers, T. R., de Jager, S. C. A., Pasterkamp, G. & Goumans, M. J. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vascul. Pharmacol. 106, 1–8 (2018).
Evrard, S. M. et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 7, 11853 (2016).
Chen, P.-Y. et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2, 1684–1696 (2012).
Hu, S. et al. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: an in vivo optical coherence tomography study. Catheter. Cardiovasc. Interv. 89, 592–600 (2017).
Xing, L. et al. EROSION study (Effective Anti-Thrombotic Therapy without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): a 1-year follow-up report. Circ. Cardiovasc. Interv. 10, e005860 (2017).
Ozaki, Y. et al. Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy. Eur. Heart J. 32, 2814–2823 (2011).
Subirana, I. et al. Prediction of coronary disease incidence by biomarkers of inflammation, oxidation, and metabolism. Sci. Rep. 8, 3191 (2018).
Bittner, D. O. et al. Coronary computed tomography angiography–specific definitions of high-risk plaque features improve detection of acute coronary syndrome. Circ. Cardiovasc. Imaging 11, e007657 (2018).
Sevakula, R. K. et al. State-of-the-art machine learning techniques aiming to improve patient outcomes pertaining to the cardiovascular system. J. Am. Heart Assoc. 9, e013924 (2020).
Yang, S. et al. Deep learning segmentation of major vessels in X-ray coronary angiography. Sci. Rep. 9, 16897 (2019).
Cho, H. et al. Angiography-based machine learning for predicting fractional flow reserve in intermediate coronary artery lesions. J. Am. Heart Assoc. 8, e011685 (2019).
Acknowledgements
A.C.F. is supported by a grant T32HL007208 from the National Heart, Lung, and Blood Institute. I.-K.J.’s research is supported by the Allan Grey Fellowship Fund in Cardiology and by Mr. and Mrs. Michael and Kathryn Park.
Author information
Authors and Affiliations
Contributions
A.C.F. and I.-K.J. researched data for the article, discussed its content and wrote the manuscript. I.-K.J. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.C.F. is a consultant and holds equity in Goodpath, which was not involved in the writing of this article. I.-K.J. has received educational grants from Abbott Vascular and a consulting fee from Svelte Medical.
Additional information
Peer review information
Nature Reviews Cardiology thanks H. Garcia-Garcia, P. Stone and R. Virmani for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Optical coherence tomography
-
(OCT). Intravascular diagnostic modality used during cardiac catheterization. Near-infrared light is used to create images of the coronary artery and atherosclerotic plaque from inside the vessel.
- Non-ST-segment elevation myocardial infarction
-
(Non-STEMI). A myocardial infarction that does not have ST-segment elevation on the 12-lead electrocardiogram.
- STEMI
-
A myocardial infarction characterized by complete occlusion of a coronary artery by a thrombus and by ST-segment elevation on the 12-lead electrocardiogram.
- Endothelial shear stress
-
The tangential stress generated by friction caused by flowing blood on the endothelial surface of the vessel wall.
- SYNTAX score
-
Angiographic grading tool to determine the complexity of coronary artery disease, dependent on both the number and the characteristics of stenoses in the coronary artery tree.
- Gensini score
-
Angiographic grading tool to determine the complexity of coronary artery disease that accounts for the degree and location of arterial stenoses.
- ACC/AHA lesion classification
-
Classifies lesion types by complexity; includes type A (simple lesions), type B (moderate lesions) and type C (complex lesions). Takes into consideration several factors such as the length of the lesion, its location (such as major branch involvement), the tortuosity of the vessel and the angulation required to access the vessel, among others.
- Oscillatory shear index
-
The ratio between backwards and forwards shear stress; a marker of flow reversal.
- Thrombolysis In Myocardial Infarction
-
(TIMI). The TIMI grade flow is a scoring system for the levels of coronary blood flow distal to a stenosis. TIMI grade 0 indicates no anterograde flow, grade 1 indicates faint antegrade flow and incomplete filling of the distal coronary artery, grade 2 flow indicates delayed or sluggish antegrade flow and grade 3 indicates normal flow.
Rights and permissions
About this article
Cite this article
Fahed, A.C., Jang, IK. Plaque erosion and acute coronary syndromes: phenotype, molecular characteristics and future directions. Nat Rev Cardiol 18, 724–734 (2021). https://doi.org/10.1038/s41569-021-00542-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00542-3
This article is cited by
-
Blood Flow Energy Identifies Coronary Lesions Culprit of Future Myocardial Infarction
Annals of Biomedical Engineering (2024)
-
Diagnosis of coronary layered plaque by deep learning
Scientific Reports (2023)
-
A novel deep learning model for a computed tomography diagnosis of coronary plaque erosion
Scientific Reports (2023)
-
Machine Learning in Invasive and Noninvasive Coronary Angiography
Current Atherosclerosis Reports (2023)
-
Impacts of Non-alcoholic Fatty Liver Disease on Acute Coronary Syndrome: Evidence and Controversies
Current Atherosclerosis Reports (2023)