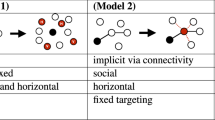
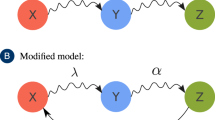
Abstract
From ‘fake news’ to innovative technologies, many contagions spread as complex contagions via a process of social reinforcement, where multiple exposures are distinct from prolonged exposure to a single source1. Contrarily, biological agents such as Ebola or measles are typically thought to spread as simple contagions2. Here, we demonstrate that these different spreading mechanisms can have indistinguishable population-level dynamics once multiple contagions interact. In the social context, our results highlight the challenge of identifying and quantifying spreading mechanisms, such as social reinforcement3, in a world where an innumerable number of ideas, memes and behaviours interact. In the biological context, this parallel allows the use of complex contagions to effectively quantify the non-trivial interactions of infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data represented in Figs. 1–4 are available as Source Data. Raw data and processing scripts are available online (https://github.com/jg-you/complex-coinfection-inference/; https://github.com/Emergent-Epidemics/Puerto_Rico_Arbovirus_Data).
Code availability
The inference software is available online (https://github.com/jg-you/complex-coinfection-inference/).
References
Lehmann S. and Ahn Y.-Y. Complex Spreading Phenomena in Social Systems (Springer, 2018).
Anderson R. M. and May R. M. Infectious Diseases of Humans: Dynamics and Control (Oxford Univ. Press, 1992).
Centola, D. The spread of behavior in an online social network experiment. Science 329, 1194–1197 (2010).
Plan of Action for Maintaining Measles, Rubella, and Congenital Rubella Syndrome Elimination in the Region of the Americas: Final Report (PAHO, 2016); http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=35681&Itemid=270&lang=en
Dabbagh, A. et al. Progress toward regional measles elimination worldwide, 2000–2017. Morb. Mortal. Wkly Rep. 67, 1323 (2018).
Fraser, B. Measles outbreak in the Americas. Lancet 392, 373 (2018).
Elidio, G. A. et al. Measles outbreak: preliminary report on a case series of the first 8,070 suspected cases, Manaus, Amazonas state, Brazil, February to November 2018. Eurosurveillance 24, 1800663 (2019).
Friedrich, M. Measles cases rise around the globe. J. Am. Med. Assoc. 321, 238–238 (2019).
Thornton, J. Measles cases in Europe tripled from 2017 to 2018. Br. Med. J. 364, 1634 (2019).
Measles Cases and Outbreaks (US CDC, accessed 18 February 2019); https://www.cdc.gov/measles/cases-outbreaks.html
Majumder, M. S., Cohn, E. L., Mekaru, S. R., Huston, J. E. & Brownstein, J. S. Substandard vaccination compliance and the 2015 measles outbreak. J. Am. Med. Assoc. Pediatr. 169, 494–495 (2015).
Phadke, V. K., Bednarczyk, R. A., Salmon, D. A. & Omer, S. B. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. J. Am. Med. Assoc. 315, 1149–1158 (2016).
Melegaro, A. Measles vaccination: no time to rest. Lancet Glob. Health 7, e282–e283 (2019).
Paniz-Mondolfi, A. et al. Resurgence of vaccine-preventable diseases in Venezuela as a regional public health threat in the Americas. Emerg. Infect. Dis. 25, 625–632 (2019).
Salmon, D. A. et al. Health consequences of religious and philosophical exemptions from immunization laws: individual and societal risk of measles. J. Am. Med. Assoc. 282, 47–53 (1999).
Papachrisanthou, M. M. & Davis, R. L. The resurgence of measles, mumps, and pertussis. J. Nurse Pract. 15, 391–395 (2019).
McHale, P., Keenan, A. & Ghebrehewet, S. Reasons for measles cases not being vaccinated with MMR: investigation into parents’ and carers’ views following a large measles outbreak. Epidemiol. Infect. 144, 870–875 (2016).
Sansonetti, P. J. Measles 2018: a tale of two anniversaries. EMBO Mol. Med. 10, e9176 (2018).
Mavragani, A. & Ochoa, G. The Internet and the anti-vaccine movement: tracking the 2017 EU measles outbreak. Big Data Cogn. Comput. 2, 2 (2018).
Van Mieghem, P. & Van de Bovenkamp, R. Non-Markovian infection spread dramatically alters the susceptible–infected–susceptible epidemic threshold in networks. Phys. Rev. Lett. 110, 108701 (2013).
Pastor-Satorras, R., Castellano, C. & Van Mieghem, P. Epidemic processes in complex networks. Rev. Mod. Phys. 87, 925 (2015).
Ross R. The Prevention of Malaria (Dutton, 1910).
Lipsitch, M., Cohen, T., Murray, M. & Levin, B. R. Antiviral resistance and the control of pandemic influenza. PLoS Med. 4, e15 (2007).
Centola, D. & Macy, M. Complex contagions and the weakness of long ties. Am. J. Sociol. 113, 702–734 (2007).
Weng, L., Menczer, F. & Ahn, Y.-Y. Virality prediction and community structure in social networks. Sci. Rep. 3, 2522 (2013).
Mønsted, B., Sapieżyński, P., Ferrara, E. & Lehmann, S. Evidence of complex contagion of information in social media: an experiment using Twitter bots. PloS ONE 12, e0184148 (2017).
Liu, W.-m, Levin, S. A. & Iwasa, Y. Influence of nonlinear incidence rates upon the behavior of SIRS epidemiological models. J. Math. Biol. 23, 187–204 (1986).
Dodds, P. S. & Watts, D. J. Universal behavior in a generalized model of contagion. Phys. Rev. Lett. 92, 218701 (2004).
Hébert-Dufresne, L., Patterson-Lomba, O., Goerg, G. M. & Althouse, B. M. Pathogen mutation modeled by competition between site and bond percolation. Phys. Rev. Lett. 110, 108103 (2013).
O’Sullivan, D. J., O’Keeffe, G. J., Fennell, P. G. & Gleeson, J. P. Mathematical modeling of complex contagion on clustered networks. Front. Phys. 3, 71 (2015).
Funk, S. & Jansen, V. A. Interacting epidemics on overlay networks. Phys. Rev. E 81, 036118 (2010).
Marceau, V., Noël, P.-A., Hébert-Dufresne, L., Allard, A. & Dubé, L. J. Modeling the dynamical interaction between epidemics on overlay networks. Phys. Rev. E 84, 026105 (2011).
Bauch, C. T. & Galvani, A. P. Social factors in epidemiology. Science 342, 47–49 (2013).
Fu, F., Christakis, N. A. & Fowler, J. H. Dueling biological and social contagions. Sci. Rep. 7, 43634 (2017).
Hébert-Dufresne, L. & Althouse, B. M. Complex dynamics of synergistic coinfections on realistically clustered networks. Proc. Natl Acad. Sci. USA 112, 10551–10556 (2015).
Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970 (2008).
Althouse, B. et al. Identifying transmission routes of Streptococcus pneumoniae and sources of acquisitions in high transmission communities. Epidemiol. Infect. 145, 2750–2758 (2017).
Nickbakhsh, S. et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl Acad. Sci. USA 116, 27142–27150 (2019).
Mair, C. et al. Estimation of temporal covariances in pathogen dynamics using Bayesian multivariate autoregressive models. PLoS Comput. Biol. 15, e1007492 (2019).
Strathdee, S. A. & Stockman, J. K. Epidemiology of HIV among injecting and noninjecting drug users: current trends and implications for interventions. Curr. HIV/AIDS Rep. 7, 99–106 (2010).
Volz, E., Frost, S. D., Rothenberg, R. & Meyers, L. A. Epidemiological bridging by injection drug use drives an early HIV epidemic. Epidemics 2, 155–164 (2010).
Allard, A., Althouse, B. M., Scarpino, S. V. & Hébert-Dufresne, L. Asymmetric percolation drives a double transition in sexual contact networks. Proc. Natl Acad. Sci. USA 114, 8969–8973 (2017).
Wearing, H. J., Rohani, P. & Keeling, M. J. Appropriate models for the management of infectious diseases. PLoS Med. 2, e174 (2005).
Shrestha, S. et al. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci. Transl. Med. 5, 191ra84–191ra84 (2013).
Liu, Q.-H. et al. Measurability of the epidemic reproduction number in data-driven contact networks. Proc. Natl Acad. Sci. USA 115, 12680–12685 (2018).
Lorenz-Spreen, P., Mønsted, B. M., Hövel, P. & Lehmann, S. Accelerating dynamics of collective attention. Nat. Commun. 10, 1759 (2019).
Halstead, S. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265, 739 (1977).
Halstead, S. B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60, 421–467 (2003).
Hill, A. L., Rand, D. G., Nowak, M. A. & Christakis, N. A. Emotions as infectious diseases in a large social network: the SISa model. Proc. R. Soc. B 277, 3827–3835 (2010).
Janssen, H.-K., Müller, M. & Stenull, O. Generalized epidemic process and tricritical dynamic percolation. Phys. Rev. E 70, 026114 (2004).
Carpenter, B. et al. Stan: A probabilistic programming language. J. Stat. Softw. https://doi.org/10.18637/jss.v076.i01 (2017).
Yang, J. & Leskovec, J. Patterns of temporal variation in online media. In Proc. 4th ACM International Conference on Web Search and Data Mining (eds King, I. & Li, H.) 177–186 (ACM, 2011).
Dengue Forecasting Project (NOAA, accessed 10 March 2019); https://dengueforecasting.noaa.gov/
Acknowledgements
L.H.-D. acknowledges support from the National Science Foundation grant DMS-1829826 and the National Institutes of Health 1P20 GM125498-01 Centers of Biomedical Research Excellence Award. S.V.S. acknowledges support from start-up funds provided by Northeastern University. J.-G.Y. is supported by a James S. McDonnell Postdoctoral Fellowship. We also thank A. Allard, B. M. Althouse, M. Newman, G. Cantwell and A. Kirkley for insightful discussions as well as J. Burkardt for sharing his Bernstein polynomial implementation.
Author information
Authors and Affiliations
Contributions
L.H.-D. conceived the study and conducted the simulations. J.-G.Y. designed and implemented the inference procedure. L.H.-D. and J.-G.Y. performed the calculations. S.V.S. compiled the empirical data. All authors interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Physics thanks Nicholas Christakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, details and benchmarks for the inference procedure and numerical simulations, and additional computational tests of the results.
Source data
Source Data Fig. 1
Computational Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical and computational Source Data.
Source Data Fig. 4
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Hébert-Dufresne, L., Scarpino, S.V. & Young, JG. Macroscopic patterns of interacting contagions are indistinguishable from social reinforcement. Nat. Phys. 16, 426–431 (2020). https://doi.org/10.1038/s41567-020-0791-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-020-0791-2
This article is cited by
-
Effects of experts on the coupling dynamics of complex contagion of awareness and epidemic spreading
Nonlinear Dynamics (2024)
-
Assessing individual risk and the latent transmission of COVID-19 in a population with an interaction-driven temporal model
Scientific Reports (2023)
-
Modeling time evolving COVID-19 uncertainties with density dependent asymptomatic infections and social reinforcement
Scientific Reports (2022)
-
Deep learning of contagion dynamics on complex networks
Nature Communications (2021)
-
Assessing the risks of ‘infodemics’ in response to COVID-19 epidemics
Nature Human Behaviour (2020)