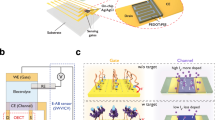
Abstract
Reusable point-of-care biosensors offer a cost-effective solution for serial biomarker monitoring, addressing the critical demand for tumour treatments and recurrence diagnosis. However, their realization has been limited by the contradictory requirements of robust reusability and high sensing capability to multiple interactions among transducer surface, sensing probes and target analytes. Here we propose a drug-mediated organic electrochemical transistor as a robust, reusable epidermal growth factor receptor sensor with striking sensitivity and selectivity. By electrostatically adsorbing protonated gefitinib onto poly(3,4-ethylenedioxythiophene):polystyrene sulfonate and leveraging its strong binding to the epidermal growth factor receptor target, the device operates with a unique refresh-in-sensing mechanism. It not only yields an ultralow limit-of-detection concentration down to 5.74 fg ml−1 for epidermal growth factor receptor but, more importantly, also produces an unprecedented regeneration cycle exceeding 200. We further validate the potential of our devices for easy-to-use biomedical applications by creating an 8 × 12 diagnostic drug-mediated organic electrochemical transistor array with excellent uniformity to clinical blood samples.
This is a preview of subscription content, access via your institution
Access options





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the article Source data and its Supplementary Information. Source data are provided with this paper. Other related raw data are available from the corresponding authors on reasonable request. The macromolecular structures of EGFR, AMPD2 and tubulin used in this study are available via the wwPDB database.
Code availability
The code that supports the theoretical plots within this paper is available from the corresponding authors upon reasonable request.
References
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Pal, A., Shinde, R., Miralles, M. S., Workman, P. & de Bono, J. Applications of liquid biopsy in the pharmacological audit trail for anticancer drug development. Nat. Rev. Clin. Oncol. 18, 454–467 (2021).
Alix-Panabières, C. & Pantel, K. Clinical prospects of liquid biopsies. Nat. Biomed. Eng. 1, 0065 (2017).
McNerney, R. & Daley, P. Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nat. Rev. Microbiol. 9, 204–213 (2011).
Land, K. J., Boeras, D. I., Chen, X. S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019).
Najjar, D. et al. A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 6, 968–978 (2022).
Cohen, L. & Walt, D. R. Highly sensitive and multiplexed protein measurements. Chem. Rev. 119, 293–321 (2019).
Manoli, K. et al. Printable bioelectronics to investigate functional biological interfaces. Angew. Chem. Int. Ed. 54, 12562–12576 (2015).
Aleman, J., Kilic, T., Mille, L. S., Shin, S. R. & Zhang, Y. S. Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat. Protoc. 16, 2564–2593 (2021).
Kim, J., Campbell, A. S., de Avila, B. E. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Naresh, V. & Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21, 1109 (2021).
Goode, J. A., Rushworth, J. V. & Millner, P. A. Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31, 6267–6276 (2015).
Sempionatto, J. R., Lasalde-Ramirez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–E2302 (2017).
Bryan, T., Luo, X., Bueno, P. R. & Davis, J. J. An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood. Biosens. Bioelectron. 39, 94–98 (2013).
Huang, J. et al. Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection. Nat. Mater. 21, 598–607 (2022).
Frutiger, A. et al. Nonspecific binding-fundamental concepts and consequences for biosensing applications. Chem. Rev. 121, 8095–8160 (2021).
Ali, M. A. et al. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 33, e2006647 (2021).
Shen, H. et al. Molecular antenna tailored organic thin-film transistors for sensing application. Mater. Horiz. 5, 240–247 (2018).
Kitao, Y. et al. The discovery of 3,3-dimethyl-1,2,3,4-tetrahydroquinoxaline-1-carboxamides as AMPD2 inhibitors with a novel mechanism of action. Bioorg. Med. Chem. Lett. 80, 129110 (2023).
Sharafeldin, M. et al. Detecting cancer metastasis and accompanying protein biomarkers at single cell levels using a 3D-printed microfluidic immunoarray. Biosens. Bioelectron. 171, 112681 (2021).
Huse, M. & Kuriyan, J. The conformational plasticity of protein kinases. Cell 109, 275–282 (2002).
Xie, T., Saleh, T., Rossi, P. & Kalodimos, C. G. Conformational states dynamically populated by a kinase determine its function. Science 370, 189 (2020).
Park, J., McDonald, J. J., Petter, R. C. & Houk, K. N. Molecular dynamics analysis of binding of kinase inhibitors to WT EGFR and the T790M mutant. J. Chem. Theory Comput. 12, 2066–2078 (2016).
Friedlein, J. T., McLeod, R. R. & Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 63, 398–414 (2018).
Romele, P., Ghittorelli, M., Kovács-Vajna, Z. M. & Torricelli, F. Ion buffering and interface charge enable high performance electronics with organic electrochemical transistors. Nat. Commun. 10, 3044 (2019).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Tybrandt, K., Zozoulenko, I. V. & Berggren, M. Chemical potential–electric double layer coupling in conjugated polymer–polyelectrolyte blends. Sci. Adv. 3, eaao3659 (2017).
Herbst, R. S., Fukuoka, M. & Baselga, J. Gefitinib—a novel targeted approach to treating cancer. Nat. Rev. Cancer 4, 956–965 (2004).
da Cunha Santos, G., Shepherd, F. A. & Tsao, M. S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 6, 49–69 (2011).
Dinish, U. S. et al. Highly sensitive SERS detection of cancer proteins in low sample volume using hollow core photonic crystal fiber. Biosens. Bioelectron. 33, 293–298 (2012).
Kumar, R. R., Meenakshi, A. & Sivakumar, N. Enzyme immunoassay of human epidermal growth factor receptor (hEGFR). Hum. Antibodies 10, 143–147 (2001).
Chen, J.-C., Sadhasivam, S. & Lin, F.-H. Label free gravimetric detection of epidermal growth factor receptor by antibody immobilization on quartz crystal microbalance. Process Biochem. 46, 543–550 (2011).
Wegner, K. D. et al. Nanobodies and nanocrystals: highly sensitive quantum dot-based homogeneous FRET immunoassay for serum-based EGFR detection. Small 10, 734–740 (2014).
Wang, Y. et al. Low sample volume origami-paper-based graphene-modified aptasensors for label-free electrochemical detection of cancer biomarker-EGFR. Microsyst. Nanoeng. 6, 32 (2020).
Li, R. et al. Electrochemical biosensor for epidermal growth factor receptor detection with peptide ligand. Electrochim. Acta 109, 233–237 (2013).
Vasudev, A., Kaushik, A. & Bhansali, S. Electrochemical immunosensor for label free epidermal growth factor receptor (EGFR) detection. Biosens. Bioelectron. 39, 300–305 (2013).
Elshafey, R., Tavares, A. C., Siaj, M. & Zourob, M. Electrochemical impedance immunosensor based on gold nanoparticles–protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue. Biosens. Bioelectron. 50, 143–149 (2013).
Altintas, Z., Kallempudi, S. S. & Gurbuz, Y. Gold nanoparticle modified capacitive sensor platform for multiple marker detection. Talanta 118, 270–276 (2014).
Shen, Y. C. et al. IGZO thin film transistor biosensors functionalized with ZnO nanorods and antibodies. Biosens. Bioelectron. 54, 306–310 (2014).
Wang, Z. et al. A flexible and regenerative aptameric graphene–Nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv. Funct. Mater. 31, 2005958 (2020).
Ferguson, B. S. et al. Integrated microfluidic electrochemical DNA sensor. Anal. Chem. 81, 6503–6508 (2009).
Bronder, T. S., Poghossian, A., Jessing, M. P., Keusgen, M. & Schoning, M. J. Surface regeneration and reusability of label-free DNA biosensors based on weak polyelectrolyte-modified capacitive field-effect structures. Biosens. Bioelectron. 126, 510–517 (2019).
Wang, S. et al. An automated microfluidic system for single-stranded DNA preparation and magnetic bead-based microarray analysis. Biomicrofluidics 9, 024102 (2015).
Pursey, J. P., Chen, Y., Stulz, E., Park, M. K. & Kongsuphol, P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuators B 251, 34–39 (2017).
Xu, S. et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 8, 14902 (2017).
Fenoy, G. E., Marmisolle, W. A., Azzaroni, O. & Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 148, 111796 (2020).
Zhang, X. et al. Photoinduced regeneration of an aptamer-based electrochemical sensor for sensitively detecting adenosine triphosphate. Anal. Chem. 90, 4968–4971 (2018).
Liu, N. et al. Regenerative field effect transistor biosensor for in vivo monitoring of dopamine in fish brains. Biosens. Bioelectron. 188, 113340 (2021).
Chen, Y., Yang, Y. & Tu, Y. An electrochemical impedimetric immunosensor for ultrasensitive determination of ketamine hydrochloride. Sens. Actuators B Chem. 183, 150–156 (2013).
Xu, M., Luo, X. & Davis, J. J. The label free picomolar detection of insulin in blood serum. Biosens. Bioelectron. 39, 21–25 (2013).
Liu, Y. L. et al. Mechanical distension induces serotonin release from intestine as revealed by stretchable electrochemical sensing. Angew. Chem. Int. Ed. 59, 4075–4081 (2020).
Yoo, H. et al. Magnetically-refreshable receptor platform structures for reusable nano-biosensor chips. Nanotechnology 27, 045502 (2016).
Ariffin, E. Y. et al. An ultrasensitive hollow-silica-based biosensor for pathogenic Escherichia coli DNA detection. Anal. Bioanal. Chem. 410, 2363–2375 (2018).
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation (22125504, 61971396 and 22021002), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0520200), the K. C. Wong Education Foundation (GJTD-2020-02), Key Research Program of Frontier Sciences CAS (ZDBS-LYSLH034), the Beijing Municipal Natural Science Foundation (Z220025) and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
F.Z. and C.-a.D. conceived and led the study. Z.J., D.Y. and F.Z. performed the experiments. Z.J., D.Y., L.X., F.Z. and C.-a.D. conducted the data analyses and mechanism discussion. J.Y. and Q.P. carried out the molecular dynamics simulation. X.D. and Y.Z. performed the XPS and UPS detection. Z.H., Y.M. and S.W. helped with the concept modification. Q.X. and J.L. provided the clinical blood samples and clinical testing. All the authors contributed to paper organization and preparation. Z.J., D.Y. and L.X. contributed equally to the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Dirk Mayer, Y. Shrike Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–44, Tables 1–9, caption for Video 1 and References.
Supplementary Video 1
A POC toolbox based on DM-OECT for blood detection.
Source data
Source Data Fig. 2
Source data for Fig. 2a–e. Source Data Fig. 3 Source data for Fig. 3a–g. Source Data Fig. 4 Source data for Fig. 4b–e. Source Data Fig. 5 Source data for Fig. 5b–e,g.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Z., Ye, D., Xiang, L. et al. A drug-mediated organic electrochemical transistor for robustly reusable biosensors. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01970-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01970-5
This article is cited by
-
A refresh-in-sensing reusable biosensor
Nature Materials (2024)