Abstract
Essential genes are commonly assumed to function in basic cellular processes and to change slowly. However, it remains unclear whether all essential genes are similarly conserved or if their evolutionary rates can be accelerated by specific factors. To address these questions, we replaced 86 essential genes of Saccharomyces cerevisiae with orthologues from four other species that diverged from S. cerevisiae about 50, 100, 270 and 420 Myr ago. We identify a group of fast-evolving genes that often encode subunits of large protein complexes, including anaphase-promoting complex/cyclosome (APC/C). Incompatibility of fast-evolving genes is rescued by simultaneously replacing interacting components, suggesting it is caused by protein co-evolution. Detailed investigation of APC/C further revealed that co-evolution involves not only primary interacting proteins but also secondary ones, suggesting the evolutionary impact of epistasis. Multiple intermolecular interactions in protein complexes may provide a microenvironment facilitating rapid evolution of their subunits.
Similar content being viewed by others
Main
Essential genes are a group of genes required for cell survival in normal growth conditions. A broad spectrum of species shares a large proportion of their essential gene repertoires1,2. In humans, many genetic diseases have been linked to mutations in essential genes3. Therefore, understanding how essential genes change over the course of evolution is important to both basic and medical sciences. Essential genes are mainly involved in core cellular functions that arose early in biotic evolutionary history, so they are commonly assumed to be under strong purifying selection to maintain their physiological functions4. This assumption is supported by a genome-wide analysis of gene essentiality between two distantly related yeast species, Schizosaccharomyces pombe and Saccharomyces cerevisiae, which revealed a high degree of conservation among essential genes despite ~420 Myr of divergence2. However, in a recent survey of 414 yeast essential gene mutants, only less than half of them could be complemented by human orthologues5. Using a similar approach, 31 of 51 tested bacterial orthologues could substitute for yeast essential genes6. Obviously, even though essential physiological functions appear conserved, the underlying molecular or regulatory mechanisms vary sufficiently to result in genetic incompatibility between species.
How can a gene maintain its physiological function and yet change its molecular properties to a level causing genetic incompatibility? More than 100 years ago, William Bateson introduced the concept of ‘epistasis’ to describe the puzzling phenotypic variation observed when the same genetic locus was combined with different genetic backgrounds7. According to the hypothesis of epistasis, how a gene behaves (and is selected) is determined by the interaction between the gene and its current genetic background. For example, a ‘disease-causing’ mutation may or may not result in pathogenesis, depending on whether compensatory mutations exist in the genetic background8. Non-additive interactions under epistasis between two mutations can lead to phenotypes weaker or stronger than expected according to summation of both individual mutation effects9. Thus, theoretically, a conserved physiological function can result from a combination of epistatically interacting non-neutral mutations at the molecular level. However, when the genetic architecture is changed, these mutations may reveal deleterious effects on cell fitness, as observed in orthologue incompatibility between species10. Moreover, it raises an intriguing hypothesis that a gene with more genetic interactions may be able to accumulate a broader spectrum of mutations while maintaining a conserved function.
Although epistasis has long been used by geneticists and evolutionary biologists to describe complicated interactions between genetic loci, it is not until recently that biologists started to gain insight into its underlying molecular mechanisms. Epistasis within molecules often involves mutations affecting the conformation or stability of a protein or RNA11,12. Moreover, it has been shown to impact mutation fixation and therefore the evolutionary path of proteins12,13,14. However, the mechanisms of epistasis that operate between molecules appear to be more complex and remain largely unresolved. Redundancy of genes or pathways, network topology, physical interactions of molecules and molecular chaperons have all been suggested to play a role in intermolecular epistasis15. Nonetheless, it is not clear how each mechanism contributes to long-term evolution, and even less so for higher-order epistasis that involves multiple interactions16. As epistatically interacting genetic loci have been observed or widely implicated in quantitative complex traits17, hybrid vigour, speciation18,19 and the evolution of sex20, detailed characterization of intermolecular epistasis would provide a better understanding of various fundamental aspects of evolutionary biology, including the evolution of essential genes.
To know how essential genes diverge between different species and whether there are specific patterns during essential gene evolution, we systematically replaced 86 essential genes in S. cerevisiae with corresponding orthologues from species that had diverged from it over different timeframes. Our results show that essential genes exhibit a wide range of variation in their evolutionary trajectories. By analysing the orthologous genes that have quickly become incompatible with the S. cerevisiae background, we discovered that intermolecular epistasis plays a key role in their evolution and that conserved physiological functions are maintained by co-evolution of interacting components. Finally, we investigated the fast-evolving anaphase-promoting complex/cyclosome (APC/C) to illustrate how multiple interactions between different components in a large protein complex have influenced the evolutionary pattern of essential genes.
Broad evolutionary trajectory variation in essential genes
To examine how essential genes change during the course of evolution, we replaced essential genes in S. cerevisiae with orthologues from other yeast species (Fig. 1a and Extended Data Fig. 1; see Methods for details). If an orthologue could not rescue cell viability in an essential gene-deleted S. cerevisiae mutant, it indicates that the orthologous essential gene has changed to such an extent that it is no longer compatible with the S. cerevisiae genomic background. We tested orthologous essential genes from four ascomycete yeast species—Naumovozyma castellii (Ncas), Kluyveromyces lactis (Klac), Yarrowia lipolytica (Ylip) and S. pombe (Spom)—that are estimated to have diverged from their common ancestor with S. cerevisiae (Scer) about 50, 100, 270 and 420 Myr ago (Ma), respectively21,22. By covering a range of species, we anticipated revealing the evolutionary patterns of essential genes.
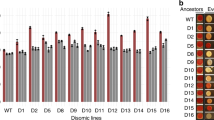
a, Orthologues from four additional Ascomycota species were used to study the evolution of essential genes. These four species diverged from S. cerevisiae over different timeframes, allowing us to characterize when the orthologues had become incompatible. To measure compatibility, S. cerevisiae essential genes were replaced by orthologues from the other four species and then cell viability was examined. Depending on the pattern of compatibility between orthologues, we classified the tested essential genes into four types: static, gradual, punctuate and fast. b, A total of 12 to 39% of tested orthologues are incompatible with the S. cerevisiae background. We performed a chi-square test to examine whether some species have more incompatible orthologues than the others and incompatible gene numbers are significantly different between different species (chi-square test, n = 340, P = 2.2 × 10−5). c, Distributions of essential genes exhibiting different evolutionary patterns. Only genes for which all four orthologues were tested are included in this analysis. Left: percentage of genes with different evolutionary patterns. Middle: the species when first incompatible orthologues were observed in the gradual group. Right: species-specific incompatibility in the punctuate group. Numbers of genes are shown in parentheses. d, The fast-type genes have the highest evolutionary rates between S. cerevisiae and N. castellii. Boxplots indicate median (middle line), 25th and 75th percentile (box), and minimum and maximum (whiskers). Distributions with different letters (above each boxplot) are significantly different from each other (fast: n = 8; gradual: n = 25; puncutate: n = 7; static: n = 44, two-sided Mann–Whitney U test, P values <0.05). See also the Source data for detailed statistical information. e, Most tested genes exhibit consistent evolutionary characteristics in all five species. Non-synonymous substitution rates (Ka) of tested orthologues from all species pairs were calculated (Supplementary Table 5) and high correlations of Ka between different species pairs were observed (one-tailed Spearman’s rank correlation, ρ = 0.85–0.99, P = 9.29 × 10−24–6.10 × 10−66; see Supplementary Table 6 for all P values). Spearman’s correlation coefficients are shown in the figure.
In S. cerevisiae, about 1,000 genes (18% of the genome) are known to be essential for cell viability of the lab strain in rich medium1. We selected 86 essential genes involved in various cellular functions for a compatibility test (Supplementary Table 1). Candidate genes with high and low sequence divergence were slightly overrepresented because we speculated that these two types of gene might reveal different patterns at the level of protein function (Extended Data Fig. 2 and Supplementary Table 2). In this study, we only focused on the evolution of protein coding regions because it is an experimentally underexplored topic. In addition, previous studies and our pilot experiments have shown that the regulation of gene expression could change quickly even among closely related species (Supplementary Table 3)23,24. Therefore, in our initial screen, we used the Tet-Off promoter to drive both S. cerevisiae and orthologous genes, and compared their phenotypes (see Methods). In later experiments, we also used the endogenous promoters from S. cerevisiae to confirm that observed incompatibility was not caused by unbalanced stoichiometry.
When growing in rich medium, only 9% of the control strains in which an essential gene was replaced by the Tet–Scer coding sequence (CDS) copy showed more than a 20% change in growth rate, indicating that replacing the endogenous promoter of an essential gene with the Tet-Off promoter does not impose a heavy burden on the cell in most strains (Extended Data Fig. 3a and Supplementary Table 4). In contrast, when the orthologues from different species were examined in S. cerevisiae essential gene-deleted mutants, around 12 to 39% of them failed to rescue cell viability, with the orthologues from more distal donors having lower percentages of compatibility (Fig. 1b).
To further analyse the specific features of different essential genes, we classified them into four types (Supplementary Table 1 and Fig. 1a,c). The majority of tested genes (44/84 = 52%) belong to the ‘static’ type in which all four orthologues can function in the S. cerevisiae background, suggesting that these genes have retained similar molecular functions and interactions for almost half a billion years (Fig. 1c). A recent study also showed that 47% of tested human orthologues were able to substitute for essential genes in yeast cells5. Our results evidence that a large proportion of essential genes evolve slowly and are conserved across divergent lineages. The others probably reflect lineage-specific evolution. The ‘gradual’ type comprises 25 genes (25/84 = 29%) that have evolved incompatibility over the course of yeast evolution, and the incompatibility appears consistently through more distant species (including the ones that only show incompatibility in the S. pombe orthologues). Genes of the ‘punctuate’ type (7/84 = 8%) show branch-specific incompatibility not in accordance with the pattern of species divergence, perhaps reflecting specific changes to individual lineages (Fig. 1c). For example, S. cerevisiae and Y. lipolytica have different tRNA gene repertoires25, and the incompatibility of several Ylip orthologues may partly be explained by compromised translation due to differences in codon usage. In the ‘fast’ type (8/84 = 10%), none of the orthologues are compatible with the S. cerevisiae genome. To rule out the possibility that the observed incompatibility was due to unbalanced stoichiometry caused by the Tet-Off promoter, we expressed the ‘fast’-type genes of N. castellii under the endogenous promoters from S. cerevisiae. None of these constructs rescued the viability (Supplementary Table 3), indicating that incompatibility resulted from the changes in CDSs. These data suggest that either these genes are fast evolving and become incompatible in species as closely related as N. castellii and S. cerevisiae (that is, within 50 Myr), or the S. cerevisiae genome has accumulated branch-specific mutations that make other orthologues incompatible. Although our experiments could not distinguish these two possibilities, our following sequence analyses revealed that the fast-type genes also change their sequences quickly in other lineages, suggesting their general fast-evolving nature.
We compared the protein sequence identity and non-synonymous substitution rate (Ka) between these four types of essential gene to see whether the compatibility patterns are correlated with sequence divergence. Indeed, the fast group has the highest evolutionary rate and protein sequence divergence, and the static group has the lowest ones (Fig. 1d and Extended Data Fig. 4). To test whether fast evolution only occurs in the S. cerevisiae lineage, we calculated the non-synonymous substitution rate of tested orthologues from all species pairs (Supplementary Table 5) and examined the correlation of Ka between different species pairs. If the same group of genes always behave similarly in different lineages, we expected to see high correlations between them. In contrast, if the pattern is only specific to the S. cerevisiae lineage (for example, fast-evolving genes only change quickly in S. cerevisiae, but not in other species), we expected to see much lower correlations between S. cerevisiae-containing pairs and non-S. cerevisiae pairs. High correlations were observed in all pairwise comparisons (Spearman’s correlation coefficient ρ = 0.85–0.99, P = 2.09 × 10−25–2.66 × 10−69, Spearman’s rank correlation; Fig. 1e and Supplementary Table 6), suggesting that most tested genes maintain consistent evolutionary characteristics in all five species.
Next, we examined growth rates of the viable strains carrying the compatible orthologues (Extended Data Fig. 3b,c and Supplementary Table 4). S. cerevisiae strains carrying N. castellii orthologues showed similar growth rates to those hosting the control Tet–Scer copy (paired t-test, n = 63, P = 0.347). In contrast, orthologues from more distal donors resulted in lower growth rates (paired t-test, Scer versus Klac, n = 57, P = 0.026; Scer versus Ylip, n = 42, P = 0.003; Scer versus Spom, n = 41, P = 0.009; Extended Data Fig. 3b). These results indicate that many subtle differences had gradually accumulated in these distant orthologues even though they remain compatible with the S. cerevisiae genome.
Interactor co-expression rescues the incompatible orthologues
The observed orthologue incompatibility could result from changes in the function or interactions. As the primary functions of tested genes are essential for cell viability, a major functional switch is unlikely and only subtle alterations may occur. On the other hand, we found that the proteins encoded by the ‘fast’-type genes all work as functional subunits of large stable protein complexes26, including Apc1, Apc2, Apc4 and Apc5 in the APC/C27, Sec20 in the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) complex28, Taf8 in the transcription factor II D (TFIID) complex29, Spp382 in the spliceosome disassembly complex, and Cdc13 in the telomere maintenance Ctc1–Stn1–Ten1 (CST) complex30 (Supplementary Tables 1 and 7). This finding raises the possibility that phenotypic stasis may be achieved by co-evolution of interacting proteins (that is, changes in the target genes followed by complementary mutations occurring on interacting partners), leading to changes in molecular structure but not physiological function.
Under the protein co-evolution hypothesis, we expected the incompatibility of fast-evolving orthologues to be rescued by co-expression of their interacting partners from the same species. We tested this hypothesis using incompatible orthologues from N. castellii because this is the most closely related species to S. cerevisiae among those we tested and molecular changes are probably more complex in more divergent species. We selected all possible interacting partners of these incompatible genes based on published biochemical experiments (Supplementary Table 7). In six out of the eight fast-type genes, we were able to uncover compensatory partners; the incompatibilities of Ncas–Apc2, –Apc4, –Apc5, –Spp382, –Sec20 and –Taf8 were rescued by co-expressing one of their direct interacting partners, that is, Ncas–Apc11, –Apc5, –Apc4, –Ntr2, –Tip20 and –Taf10, respectively (Supplementary Table 7). The only exceptions were Ncas–Cdc13 and Ncas–Apc1. Cdc13 is a telomere binding protein, so it is possible that the incompatibility could have arisen from a mismatch between the protein and DNA motifs rather than protein–protein interactions30. Co-expressing other individual Ncas–APC/C essential components did not rescue the incompatibility of Ncas–Apc1. We speculate that it may be necessary to co-express multiple interacting partners to rescue this case of incompatibility because Apc1 is a scaffold protein that bridges different subunits of the APC/C complex.
To ensure that the observed APC/C subunit incompatibility and protein co-evolution are not a phenomenon specific to the S. cerevisiae lineage, we also tested the compatibility of Scer–Apc2 in the N. castellii background. Scer–Apc2 alone failed to rescue the viability of an N. castellii apc2 deletion mutant. However, the mutant cells became viable if we co-expressed Scer–APC2 and Scer–APC11 (Supplementary Table 8 and Methods). These results provide strong evidence that co-evolution of interacting proteins contributes substantially to the observed incompatibility, especially for the fast-type genes.
Multiple domains are involved in Ncas–Apc2 incompatibility
Next, we aimed to understand the molecular processes of the co-evolution of an essential gene and its partner. Two co-evolving pairs, Apc2–Apc11 and Apc4–Apc5, are components of the APC/C complex, which may serve as an interesting case on how intermolecular interactions influence the evolution of protein subunits in a large protein complex. APC/C regulates a variety of important cellular processes such as cell division, differentiation, genome stability, autophagy and cell death, as well as being linked to carcinogenesis31. APC/C is a large protein complex in which 9 of 13 major components are essential for S. cerevisiae cell viability. One co-evolving pair, Apc4 and Apc5, are the scaffold proteins of APC/C and the other pair, Apc2 and Apc11, are the enzymatic core of the E3 ubiquitin ligase, APC/C, which controls cell cycle progression by ubiquitinating cell cycle regulators32. Protein structural information is available for Apc2 and Apc11, as well as other APC/C components, making this a good paradigm for studying the evolution of intermolecular interactions in detail27,33.
Apc2 contains two protein-binding domains27,34. Previous biochemical and cryogenic electron microscopy structural data indicate that the amino (N)-terminal cullin domain (NTD) binds the scaffold protein Apc1 and the carboxy (C)-terminal globular domain (CTD) interacts with Apc11 and Doc1, which helps the complex to position its substrates and activators (Fig. 2a and Extended Data Fig. 5)34. We constructed two chimeric proteins, NcasScer–Apc2 and ScerNcas–Apc2, in which the NTDs of Scer–Apc2 and Ncas–Apc2 were swapped (Fig. 2b). We then tested the functionality of these chimeric proteins in the presence of Ncas–Apc11 (Fig. 2c) or Scer–Apc11 (Fig. 2d) to assess the contributions of the different domains to subunit incompatibility. Our results show that NcasScer–Apc2 could not function with Ncas–Apc11 and further suggested that the mis-interaction with Scer–Apc2 contributes to the incompatibility of Ncas–Apc11 (Fig. 2c).
a, A simplified diagram depicting the interactions between Apc2, Apc11 and other APC/C components, which we employ in subsequent figures to illustrate how the structure of APC/C is affected by substituted orthologous proteins. The CTD of Apc2 is the main interacting domain with Apc11 and the NTD of Apc2 interacts with other APC/C components. b, Construction of chimeric proteins of Scer–Apc2 and Ncas–Apc2. N. castellii (Ncas) proteins are in blue and S. cerevisiae (Scer) proteins are in pink. c, Cells with the NTD of Scer–Apc2 or Ncas–Apc2 exhibit different fitness, suggesting that the NTD of Apc2 also contributes to the stability of the whole complex. The blue complex at top right represents the Ncas–APC/C. NA, data not available due to cell inviability. Control, the apc2Δ apc11Δ shuffling strain carrying the plasmid containing Scer–APC2 and Scer–APC11. d, Cells carrying ScerNcas–Apc2 and Scer–Apc11 do not have obvious growth defects, suggesting that the incompatibility of Ncas–Apc2 is not completely due to mis-interaction between the CTD of Ncas–Apc2 and Scer–Apc11. All compatibility tests of different proteins were performed in an apc2Δ apc11Δ shuffling strain hosting plasmids carrying the orthologous or chimeric genes being driven by the Tet-Off promoter. Growth rates were normalized to that of a control strain (apc2Δ apc11Δ + Scer–APC2 Scer–APC11). Error bars represent the standard error of the mean from four independent colonies. Columns sharing the same letters are not significantly different (ɑ = 0.05, Tukey-adjusted P values). Source data and detailed statistical information are provided in the Source data. See also Extended Data Fig. 5.
However, cells carrying ScerNcas–Apc2 were viable when it was co-expressed with Scer–Apc11 (Fig. 2d), suggesting that the Ncas CTD alone is not sufficient to cause Ncas–Apc2 incompatibility. One possible explanation is that even though the Ncas CTD of ScerNcas–Apc2 has difficulties in interacting with Scer–Apc11 (Fig. 2d), the interactions between the Scer NTD of this chimeric protein and the other S. cerevisiae APC/C components may stabilize the complex structure. This hypothesis is supported by our observation that in the presence of Ncas–Apc11 (Fig. 2c), cells hosting ScerNcas–Apc2 grew better than Ncas–Apc2-hosting cells, despite the interaction interfaces between Apc11 and Apc2 (that is, Ncas–Apc11 and the CTD of Ncas–Apc2) being exactly the same in both cases. However, mis-interaction of the NTD alone is also insufficient to disrupt APC/C function (Fig. 2d). Our results suggest that the individual domains of Apc2 interact with different proteins in the APC/C complex (that is, the CTD of Apc2 with Apc11 and the NTD of Apc2 with other APC/C components) and the effect of each interaction on cell fitness depends on the other ones.
When we tested the chimeric proteins, we observed marginal growth of cells carrying Ncas–APC2 and Scer–APC11 (Fig. 2d), in contrast to the complete incompatibility of Ncas–APC2 observed in our screening experiments. We found that this inconsistency was caused by a high expression level of Scer–APC11 driven by the TetO7 promoter we used in this experiment. When we replaced tet–Scer–APC11 with a Scer–APC11 carrying an endogenous promoter (as in the screening experiment), complete incompatibility was again observed (Extended Data Fig. 6). In addition, when Scer–Apc11 was expressed from its native promoter, ScerNcas–Apc2 showed a lower growth rate than NcasScer–Apc2, with both chimeric proteins exhibiting lower fitness than Scer–Apc2. These findings support our hypothesis that interactions between the NTD of Apc2 and other APC/C components also contribute to the co-evolution of Apc2 and Apc11.
A few critical Apc11 residue changes rescue incompatibility
Compared with Apc2 (853 amino acids (a.a.) for Scer–Apc2), Apc11 is a much smaller protein (165 a.a. for Scer–Apc11), and the compatibility of Apc11 orthologues with the S. cerevisiae genome shows an interesting punctuate pattern. Whereas orthologues from less-divergent species (Ncas–Apc11 and Klac–Apc11) exhibit incompatibility, orthologues from more divergent species (Ylip–Apc11 and Spom–Apc11) are compatible (Supplementary Table 1). Comparative sequence analysis of Apc11 from different species revealed that several amino acid residues in the conserved regions of S. cerevisiae Apc11 (a.a. 3, 4, 5, 50, 56, 60 and 64) are more similar to those of Y. lipolytica and S. pombe than they are to those of more recently diverged species (Fig. 3a and Extended Data Fig. 7). These residues are located near the interaction interface with Apc2 (Extended Data Fig. 5), suggesting that Ncas–Apc11 incompatibility might result from rapid co-evolution of the Apc2–Apc11 interaction. Hereafter, the nomenclature of these seven residues follows their relative positions in the Scer–Apc11 sequence.
a, Seven residues in conserved domains of Apc11 were found to exhibit different patterns between compatible and incompatible orthologues. The amino acids shared by incompatible orthologues are in pink and those shared by compatible orthologues are in blue. Amino acids deviating from the majority of aligned orthologues are in green (Extended Data Fig. 7). b, Replacing residue 60 in Ncas–Apc11 rescues incompatibility with Scer–Apc2. Individual or multiple mutations were introduced into Ncas–APC11 to test for compatibility with Scer–Apc2. Only V60D and 4mut (quadruple substitution of residues 50, 56, 60 and 64) exhibited detectable rescue effects. Control, the apc2Δ apc11Δ shuffling strain carrying the plasmid containing Scer–APC2 and Scer–APC11. c, Replacing residue 60 in Klac–Apc11 only partially rescues incompatibility with Scer–Apc2, whereas Klac–APC11–4mut exhibits higher compatibility. d,e, Introduction of N. castellii residues into Scer–Apc11 does not result in obvious incompatibility with Scer–Apc2 (d). Nonetheless, these mutations increase the compatibility of Scer–Apc11 with Ncas–Apc2 (e), suggesting that residue 60 is crucial for the interaction between Apc11 and Apc2. All APC11 mutants and APC2 were driven by the Tet-Off promoter. Growth rates were normalized to that of the control strain. NA, data not available due to cell inviability. Error bars represent the standard error of the mean from three independent colonies. Columns sharing the same letters are not significantly different (ɑ = 0.05, Tukey-adjusted P values). Source data and detailed statistical information are provided in the Source data. Blue stars indicate the substituted residues in the interacting interface. No change means the template is the wild-type Ncas–Apc11 or Scer–Apc11.
To examine whether these divergent sites really contribute to the difference in compatibility, we substituted combinations of these seven Scer–Apc11 residues into Ncas–Apc11 and vice versa. We then tested these mutants for compatibility with Scer–Apc2. Interestingly, only replacing residue 60 in Ncas–Apc11 with the Scer–Apc11 sequence (V60D) rescued the incompatible interaction of Ncas–Apc11 with Scer–Apc2 (Fig. 3b). Replacement of residue 60 in Klac–Apc11 (S60D) also partially rescued Klac–Apc11 incompatibility with Scer–Apc2 (Fig. 3c). Furthermore, an N. castellii-like D60V mutant of Scer–Apc11 could interact with Ncas–Apc2 and improved cell growth (Fig. 3e). This D60V Scer–Apc11 mutant still interacted with Scer–Apc2 and did not cause severe growth defects (Fig. 3d), suggesting that interaction stability is not solely determined by residue 60. Together, these results suggest that mutations of residue 60 represent a permissive step allowing the interacting interface of Apc2 and Apc11 to further change.
Even though replacements of the remaining six divergent sites did not rescue Ncas–Apc11 incompatibility, some of them interacted epistatically with residue 60. The Ncas–Apc11–4mut mutant that carried a quadruple replacement (sites 50, 56, 60 and 64) exhibited weaker compatibility with Scer–Apc2 than the Ncas–Apc11–V60D mutant (Fig. 3b). In contrast, the same quadruple replacement in the Klac–Apc11–4mut mutant showed higher compatibility with Scer–Apc2 (Fig. 3c). These results indicate that Ncas–Apc11 and Klac–Apc11 have probably evolved differently despite sharing similar divergent patterns at those seven amino acid positions.
Interactions between different APC/C components are crucial
Our earlier chimeric Apc2 experiments revealed that the interaction of the NTD of Apc2 with other APC/C proteins might influence the compatibility between Apc2 and Apc11 orthologues (Fig. 2 and Extended Data Fig. 6). APC/C is a large protein complex and its stability is influenced by multiple interactions between different protein subunits. It suggests that mutations causing a weakened interaction between two subunits may be tolerated (so the complex is still functional) if other interactions of these subunits are intact. However, the same mutations may cause a drastic effect if they occur in an unstable background. To elucidate the possible background effect, we investigated how the compatibility of mutations at the interaction interface of Apc11 with Apc2 is influenced by the interaction of Apc2 with other APC/C subunits.
We examined the fitness of the Ncas–Apc11–V60D substituent in the background of Scer–Apc2 and NcasScer–Apc2, which probably has a compromised interaction with other Scer–APC/C proteins. Figure 4a showed that the compatibility of the Ncas–Apc11–V60D substituent was much reduced in the NcasScer–Apc2 background compared with the Scer–Apc2 background. Similarly, the Ncas–Apc11–4mut mutant completely failed to rescue the viability of NcasScer–Apc2-hosting cells (Fig. 4a), although it did for Scer–Apc2-hosting cells (Figs. 3b and 4a). We also observed a similar dependency when Scer–Apc11 substituents (Scer–Apc11–D60V and Scer–Apc11–4mut) were examined. Previously, no obvious defect was observed between the Scer–Apc11 substituents and Scer–Apc2 (Fig. 3d). However, we found that the compatibility of the Scer–Apc11 substituents was significantly reduced when coexisting with NcasScer–Apc2 (Fig. 4b).
a, Ncas–APC11–V60D mutants exhibit different fitness in the NcasScer–APC2 and Scer–APC2 backgrounds. The Apc2–Apc11 interacting domains remain the same in these two strain backgrounds, but the APC/C complex is less stable in the NcasScer–APC2 background due to the foreign NTD of NcasScer–Apc2. b, Scer–APC11–4mut cells exhibit growth defects in the NcasScer–APC2 background. The 4mut and D60V mutants (see Fig. 3d) show no obvious effect in the Scer–APC2 background that has a more stable APC/C structure. Growth rates were normalized to that of the control strain. NA, data not available due to cell inviability. Error bars represent the standard error of the mean from three independent colonies. Columns sharing the same letters are not significantly different (ɑ = 0.05, Tukey-adjusted P values). Source data and detailed statistical information are provided in the Source data. Blue stars indicate the substituted residues in the interacting interface. No change means the template is the wild-type Ncas–Apc11 or Scer–Apc11. c, Illustration of the possible evolutionary trajectories of a protein complex under the influence of multiple protein interactions. Mutations accumulated in the interface of different subunits will be cryptically neutral if the structure of the complex is stably maintained by other components. As long as the effect of the mutations in the interface of primary or secondary interacting proteins does not exceed the buffering capacity of the complex (represented by the red dashed line), these mutations may accumulate in the population. Moreover, these mutations may allow interacting partners to further mutate, leading to co-evolution. However, phenotypic variation (or incompatibility) will be revealed if the stability of the complex is compromised sufficiently to exceed the buffering capacity.
Together, these results show that even though the NTD of Apc2 does not directly interact with Apc11, it can influence the impacts of the mutations affecting the Apc2–Apc11 interaction. It raises a possibility that destabilizing mutations may be sustained and further influence the evolutionary trajectory of the protein complex if they occur in a background in which the structure of the complex is stably maintained (Fig. 4c).
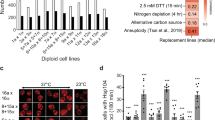
Our experimental assessment of Apc2–Apc11 co-evolution suggests that multiple protein interactions in the APC/C complex may enable it to tolerate changes in its protein interaction interfaces, allowing co-evolution of two of its subunits. To investigate how this scenario influences the evolution of other APC/C subunits, we examined orthologue compatibility for all nine essential genes of the APC/C complex in S. cerevisiae. We found that more than half of the orthologues from N. castellii exhibited incompatibility, including the core enzymatic proteins Apc2 and Apc11, as well as the structural proteins Apc1, Apc4 and Apc5 (Fig. 5). In the more divergent species K. lactis, the orthologue of Cdc23—a subunit of the TPR lobe that directly interacts with Apc5—was also incompatible. For Y. lipolytica and S. pombe, all tested orthologues except for Apc11 exhibited incompatibility (Fig. 5 and Supplementary Table 1).
Both APC/C and SCF complexes are the ubiquitin E3 ligases involved in cell cycle progression, but APC/C has far more subunits than SFC. Most APC/C core subunits evolve incompatibility between S. cerevisiae and N. castellii. In contrast, SCF core subunits remain static in all species tested (Supplementary Table 1). Orthologous APC/C subunits incompatible with S. cerevisiae are coloured purple and uncharacterized SCF subunits are coloured grey.
Co-expression of Ncas–Apc2 and Ncas–Apc11 as well as Ncas–Apc4 and Ncas–Apc5 can reverse the incompatibility of individual orthologues (Supplementary Table 8). However, we failed to see a similar reversion when co-expressing orthologous pairs (Apc2–Apc11 and Apc4–Apc5) from the more distal donor, K. lactis (Supplementary Table 8). These results suggest that, in addition to co-evolution between primary interacting subunits of the K. lactis APC/C complex, other incompatible mutations (for example, co-evolution among secondary interacting subunits) have accumulated when species further diverge.
Subunits of the Skp–Cullin–F-box complex are static
In several cases of fast-evolving genes, the molecular function of the gene products has been implicated in driving their evolution35,36. To understand whether the unique evolutionary pattern of APC/C subunits was simply driven by its molecular function, we examined the orthologue compatibility of its homologue, the Skp–Cullin–F-box (SCF) complex. SCF is another E3 ubiquitin ligase involved in cell cycle regulation34, and it has been suggested that it evolved from the same ancestral protein complex as APC/C37. However, the structure of SCF is less complex, consisting of only four proteins in S. cerevisiae. We performed orthologue replacements of Cdc53 and Rbx1, which are homologues of Apc2 and Apc11, and found that all orthologues of CDC53 and RBX1 (from the same four divergent species tested for the APC/C complex) were compatible with the S. cerevisiae genome (Fig. 5 and Supplementary Table 1). Such high conservation greatly contrasts with our findings for Apc2 and Apc11, suggesting that the molecular function is not the primary driving force for the evolutionary pattern of Apc2 and Apc11.
Discussion
A paradox in evolutionary biology is that some phenotypic traits across species do not seem to be subject to progressive changes over time and instead are shared by divergent modern species or even their fossilized ancestors. Invariant phenotypes or evolutionary stasis is often overlooked in evolutionary studies, which tend to heavily focus on traits that rapidly change in response to environmental or other factors. Moreover, the purifying selection that is widely accepted as responsible for evolutionary stasis further precludes the interests of studying the underlying mechanisms38. However, recent discoveries have challenged the conventional perception of phenotypic stasis. For example, a comparison of the vulval development of different nematode species showed that while a constant phenotype is maintained, quantitative differences in cellular responses exist between closely related Caenorhabditis species and qualitative changes in regulatory pathways occur between distantly related nematodes39. Similarly, studies of yeast have shown that, for some transcription circuits, the regulators and outputs have been conserved among different species, yet the regulatory networks have been extensively rewired40.
Evolutionary stasis can be observed at different levels of biological organization. Essential genes represent a good paradigm for studying evolutionary stasis at the gene level. Their protein sequences are more highly conserved4,41 and they are more likely to have orthologues across diverse organisms1,2. Nonetheless, recent systematic studies have revealed that a subset of essential genes can become dispensable if cells evolve bypassing mutations42,43. Genome-wide gene deletions from different strains of S. cerevisiae have also indicated that some essential genes are strain-specific44. These observations suggest that essential genes can possess diverse characteristics. Here, we demonstrate that essential genes can indeed exhibit various evolutionary patterns, with some of them suggesting that complete genetic incompatibility has occurred within 50 Myr of evolution. We further confirmed experimentally that the evolution of incompatibility in this type of essential genes is mainly driven by protein co-evolution and is probably facilitated by multiple protein interactions. This phenomenon is distinct from previously described ‘evolvable’ essential genes, the essentiality of which can be bypassed by mutations in another gene or pathway42,43. In these latter cases, whether or not an essential gene is ‘evolvable’ greatly depends on its molecular functions and the pathways involved.
Essential cellular functions are strongly constrained by purifying selection because any defect can easily lead to fitness loss or lethality of the organisms. However, selection at the gene level is more complicated. We show that protein co-evolution allows cells to tolerate extensive changes in the interaction interfaces of essential proteins without losing their conserved functions (Supplementary Table 7). Thus, as long as the structure of the entire complex is maintained, the interaction interfaces can be shaped in distinct ways among different lineages. As a consequence, seemingly neutral (or cryptic) mutations can eventually lead to incompatibility between interacting protein complex subunits (Bateson–Dobzhansky–Muller incompatibility) if their link with the arising background is disrupted18. Interestingly, when we compared static and non-static groups, non-static genes were more likely to encode protein complex subunits than static genes (34/40 versus 23/44, chi-square test, P = 0.001; Supplementary Table 1)26.
In addition to facilitating the accumulation of cryptic genetic variation, it has also been theorized that epistasis can shape the evolutionary trajectory of a gene, that is, a gene under the influence of complex epistatic effects may be able to access different fitness peaks more easily and consequently be more evolvable45,46,47. APC/C is a large protein complex that is hypothesized to have been present in the last eukaryotic common ancestor, and most of its subunits can still be found in all present-day eukaryotic lineages with few exceptions48,49. However, we found that individual orthologous subunits cannot be exchanged among closely related yeast species, revealing that components of this ancient complex can change in a short period of evolutionary time. Our analysis of APC/C subunits provides a possible scenario for how epistasis can accelerate the evolution of multiple components of an essential protein complex. Moreover, we found the ‘fast’ group genes all belong to the subunits of large protein complexes in S. cerevisiae (the sizes of which are among the top 16% of this yeast’s essential protein complexes; Supplementary Table 7). It implies that the complex environment may have an important influence on gene evolvability.
During yeast evolution, a large hybridization event happened between a K. lactis-like lineage and a Zygosaccharomyces rouxii-like lineage to generate the common ancestor of N. castellii and S. cerevisiae50,51. Following the hybridization event, many duplicated genes were lost and now they only account for less than 20% of modern yeast genomes52. It raises a possibility that maybe different duplicated copies of the APC/C components were maintained in N. castellii and S. cerevisiae, which led to observed incompatibility. However, this hypothesis is not supported by the genome synteny data. If N. castellii and S. cerevisiae keep different copies of the APC/C genes from the hybrid genome, we expect that flanking regions of the APC/C genes should have different gene contents, because two homeologous chromosomes (one from the Z. rouxii-like lineage and another from the K. lactis-like lineage) lost different genes after whole-genome duplication. However, the gene synteny data show that similar orthologous gene sets are kept in the regions flanking to APC/C genes (APC1, APC2, APC4, APC5 and APC11) in N. castellii and S. cerevisiae52, suggesting that both species retained the same copy of APC/C genes after whole-genome duplication.
Stable integrity of large protein complexes is critical for their functions and this is maintained by the interactions between multiple protein interfaces. These molecular interaction microenvironments can tolerate changes to the interacting interfaces of individual subunits or even interaction partners without causing a loss of the integrity of a protein complex or its physiological functions53. However, if the stability of the complex is compromised by further environmental or genetic perturbations, phenotypic variation would be manifested because the effect of accumulated mutations would no longer be buffered. This phenomenon is similar to the ‘capacitor’ (or ‘genetic buffering’) model proposed in studies of Hsp90, a protein implicated in facilitating population adaptation to changing environments54. One-third of the S. cerevisiae proteome has been identified as consisting of protein complex components, and the functions and structures of a large fraction of these complexes remain understudied26. Similar proportions of protein complexes are probably present in other eukaryotic organisms55. More studies are required to understand the contribution of protein complexes to long-term population adaptation and why some protein complexes can change quickly while others remain static.
Methods
Construction of plasmids
Two types of yeast single-copy plasmid were used in the orthologue compatibility test. The pRS416–Scer, pRS413–Ylip and pRS413–Spom plasmids carry the CDS of a S. cerevisiae, Y. lipolytica or S. pombe gene and its regulatory elements, including the upstream 1 kb and downstream 0.5 kb regions (Supplementary Table 9). The pRS416–Scer plasmid was used to supply the essential gene function when we deleted the genomic copy of an essential gene. pRS413–Ylip and pRS413–Spom plasmids were used to test the compatibility of the promoters when the data were compared with those of pRS413–tet–orthologue plasmids. We tested 36 orthologous genes from Y. lipolytica and S. pombe (Supplementary Table 3). Only 16 of the pRS413–Ylip or pRS413–Spom plasmids (16/36 = 44%) complemented the S. cerevisiae mutants, despite the fact that all the pRS413–tet–orthologue plasmids could complement. The pRS413–tet–orthologue plasmid contains a tetracycline responsive element (tetO7 from the pUCtetO7 plasmid), a S. cerevisiae CYC1 terminator and the CDS of a S. cerevisiae essential gene or its fungal orthologue. The orthologous CDSs were polymerase chain reaction (PCR)-amplified from the cDNA of four ascomycete yeast species: N. castellii, K. lactis, Y. lipolytica and S. pombe. As some fungal genes have multiple introns and may retain these introns in purified mRNA, we obtained the templates by de novo synthesis (GENEWIZ). The pRS413–tet–orthologue plasmids were used to test the compatibility of orthologues. In cases where expression of two plasmids was required, such as in the rescue experiment in Supplementary Table 7, pRS415 was used to generate pRS415–tet–orthologue plasmids.
Chimeric APC2 genes were constructed by swapping the NTDs of Scer–Apc2 (residues 1–429) and Ncas–Apc2 (residues 1–415). To test the effect of expression levels of Ncas–APC11, a plasmid containing the regulatory elements of Scer–APC11 and the CDS of Ncas–APC11 was also constructed. Plasmid construction was achieved by either yeast homologous recombination or In-Fusion HD Cloning Kit (Clontech). A QuikChange Multi Site-Directed Mutagenesis Kit (Agilent Technologies) was used to generate mutation clones of Scer–APC11, Ncas–APC11 and Klac–APC11. The substituted codons were optimized for expression in S. cerevisiae.
Genetic procedures of orthologue replacement
The orthologue compatibility test was conducted according to the following procedure (Extended Data Fig. 1). First, the pRS416–Scer plasmid carrying a S. cerevisiae essential gene and the URA3 marker was transformed into the JYL1821 S. cerevisiae strain, which was derived from R1158 (MATa met15–0 ura3::CMV–tTA–ura3 his3–1 leu2–0 trp1–63). Next, the genomic copy of the essential gene was deleted by transforming PCR-amplified deletion kanMX4 cassettes from the yeast essential heterozygous diploid collection (GE Dharmacon). Successful knockout strains were then transformed with the pRS413–tet–orthologue plasmids. If the orthologous gene could replace the function of the S. cerevisiae essential gene, it would allow cells to lose the pRS416–Scer plasmid and grow on 5-Fluoroorotic acid (5-FOA) plates that are toxic to cells with Ura3 activity. At least three biological repeats were performed for each replacement experiment and the results were reported only when consistent patterns were observed. Orthologues were classified as incompatible if all tested colonies could not grow on 5-FOA plates (cell survival rate <64%, binomial test, P = 0.047). Inviability of an orthologue-carrying shuffling strain on 5-FOA plates indicates that the essential gene has changed to such an extent that it cannot be replaced by its orthologue.
To generate the apc2Δ apc11Δ plasmid shuffling strain, the CDSs and regulatory elements of Scer–APC2 and Scer–APC11 were placed in the same pRS416 plasmid and then transformed into the JYL1821 strain. The genomic copies of Scer–APC2 and Scer–APC11 were deleted using kanMX6 and hphMX4 deletion cassettes, respectively.
To test the compatibility of Scer–APC2 and Scer–APC11 in the N. castellii background, the CDSs of Scer–APC2 and Scer–APC11 were fused with a highly expressed Ncas–ADH1 promoter and then inserted into the N. castellii genome to replace the endogenous Ncas–APC2 and Ncas–APC11 genes. All the genetic manipulations were done in a diploid N. castellii strain, CBS4310. After the transformants were confirmed by PCR of the insertion sites, the heterozygous replacement lines were induced to sporulation and viable spores were examined. The genetic results indicated that cells carrying only Scer–APC2 were inviable, but Scer–APC2 + Scer–APC11 double replacement cells were viable.
Maintenance and transformation of yeasts
The culture temperature was 28 °C for S. cerevisiae and 25 °C for N. castellii, K. latics, Y. lipolytica and S. pombe. We used YPD culture medium (1% yeast extract, 2% peptone, 2% glucose) for S. cerevisiae, N. castellii, K. latics and Y. lipolytica, whereas YES medium (1% yeast extract, 2% glucose) was used for S. pombe.
We used the lithium acetate method to perform transformations in S. cerevisiae56. For transformations in N. castellii, we adopted an electroporation protocol57 with slight modifications. Briefly, we resuspended 3–5 ml N. castellii overnight culture in 8 ml ddH2O, 1 ml 1 M LiOAc, 1 ml 10X TE and 250 μl 1 M DTT, and incubated it for 0.5–2 hr. Then the cell pellet was washed with 30 ml cold ddH2O and then with 5 ml cold 1 M sorbitol. After washing, the cell pellet was resuspended in 0.5 ml cold 1 M sorbitol and kept on ice before transformation. We then mixed 100 μl of cell suspension with 20 μl DNA and put the mixture in a pre-chilled 2 mm electroporation cuvette (BasicLife Bioscience). Electroporation was carried out in an ECM 630 Electroporation System (BTX) with the following settings: 1.8 kV, 200 Ω, 20 μF. After electroporation, 1 ml sorbitol was immediately added to the cell suspension and left to recover for 1 hr at 25 °C before plating on YPD plates.
RNA isolation and cDNA preparation
We used the Qiagen RNeasy Mini Kit (Qiagen) to isolate RNA from exponentially growing yeasts. The isolated RNA was reverse-transcribed to cDNA by SuperScript III Reverse Transcriptase (Invitrogen). The cDNA was used as templates for PCR and plasmid constructions (Supplementary Table 9).
Candidate gene selection and sequence analysis
We included the essential genes identified by ref. 1 and the list of essential genes from the Saccharomyces Genome Database (https://www.yeastgenome.org) for our analysis. Only genes essential to strain background S288c were considered. We focused on genes with only one orthologue in the other four selected species—N. castellii, K. lactis, Y. lipolytica and S. pombe—yielding a total of 796 essential genes.
In the first round of experiments for testing orthologue compatibility, we selected essential genes involved in various cellular functions and also some from the top or bottom ranked genes in terms of sequence conservation because we speculated that these genes might reveal different evolutionary trajectories. We successfully obtained deletion strains and orthologue plasmids for 74 genes. In the second round of experiments, we included another 12 essential genes encoding the subunits of fast-evolving protein complexes to test our hypothesis of co-evolution. Data from both experiments were combined for our analyses.
Identities and similarities between S. cerevisiae proteins and those of other yeasts were established in EMBOSS Matcher with default settings58. To calculate Ka, protein sequences were first aligned by MUSCLE (version: muscle3.8.31_i86linux64)59 and then assessed in ParaAT followed with KaKs_Calculator 2.060,61.
Growth rate measurements
Cells of different strains were cultured in 96-well YPD plates at 28 °C until saturation, that is, the cell density of each well was similar to the control strain. Then, saturated cell cultures were diluted 30-fold in fresh YPD and the growth rates were measured using an Infinite 200 (or F200) series plate reader (Tecan). The control strain was always included in each plate, and at least three replicates were measured for each sample.
Modelling the structure of S. cerevisiae APC2–APC11
Currently, there are no available protein structures of S. cerevisiae Apc2 or Apc11, so we modelled these yeast protein structures in web-based Phyre262. Among the best templates predicted by Phyre2, we chose the human APC/C structure (Protein Data Bank: 4UI9) as the template for both S. cerevisiae Apc2 and Apc11. We used PyMol visualization software (Schrodinger) to show the modelled protein structure.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated or analysed in this study are provided in the article or its Supplementary Information. Source data are provided with this paper.
References
Giaever, G. et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 (2002).
Kim, D. U. et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28, 617–623 (2010).
Park, D., Park, J., Park, S. G., Park, T. & Choi, S. S. Analysis of human disease genes in the context of gene essentiality. Genomics 92, 414–418 (2008).
Jordan, I. K., Rogozin, I. B., Wolf, Y. I. & Koonin, E. V. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res. 12, 962–968 (2002).
Kachroo, A. H. et al. Systematic humanization of yeast genes reveals conserved functions and genetic modularity. Science 348, 921–925 (2015).
Kachroo, A. H. et al. Systematic bacterialization of yeast genes identifies a near-universally swappable pathway. eLife 6, e25093 (2017).
Bateson, W. Mendel’s Principles of Heredity (Cambridge Univ. Press, 1909).
Kondrashov, A. S., Sunyaev, S. & Kondrashov, F. A. Dobzhansky–Muller incompatibilities in protein evolution. Proc. Natl Acad. Sci. USA 99, 14878–14883 (2002).
Phillips, P. C. Epistasis - the essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 9, 855–867 (2008).
Paaby, A. B. & Rockman, M. V. Cryptic genetic variation: evolution’s hidden substrate. Nat. Rev. Genet. 15, 247–258 (2014).
Kern, A. D. & Kondrashov, F. A. Mechanisms and convergence of compensatory evolution in mammalian mitochondrial tRNAs. Nat. Genet. 36, 1207–1212 (2004).
Gong, L. I., Suchard, M. A. & Bloom, J. D. Stability-mediated epistasis constrains the evolution of an influenza protein. eLife 2, e00631 (2013).
Weinreich, D. M., Delaney, N. F., DePristo, M. A. & Hartl, D. L. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312, 111–114 (2006).
Bridgham, J. T., Ortlund, E. A. & Thornton, J. W. An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461, 515–519 (2009).
Lehner, B. Molecular mechanisms of epistasis within and between genes. Trends Genet. 27, 323–331 (2011).
Weinreich, D. M., Lan, Y. H., Wylie, C. S. & Heckendorn, R. B. Should evolutionary geneticists worry about higher-order epistasis? Curr. Opin. Genet. Dev. 23, 700–707 (2013).
Bloom, J. S., Ehrenreich, I. M., Loo, W. T., Lite, T. L. & Kruglyak, L. Finding the sources of missing heritability in a yeast cross. Nature 494, 234–237 (2013).
Orr, H. A. Dobzhansky, Bateson, and the genetics of speciation. Genetics 144, 1331–1335 (1996).
Dominguez-Garcia, S., Garcia, C., Quesada, H. & Caballero, A. Accelerated inbreeding depression suggests synergistic epistasis for deleterious mutations in Drosophila melanogaster. Heredity 123, 709–722 (2019).
Kondrashov, A. S. Classification of hypotheses on the advantage of amphimixis. J. Hered. 84, 372–387 (1993).
Taylor, J. W. & Berbee, M. L. Dating divergences in the Fungal Tree of Life: review and new analyses. Mycologia 98, 838–849 (2006).
Galagan, J. E., Henn, M. R., Ma, L. J., Cuomo, C. A. & Birren, B. Genomics of the fungal kingdom: insights into eukaryotic biology. Genome Res. 15, 1620–1631 (2005).
Tirosh, I., Reikhav, S., Levy, A. A. & Barkai, N. A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324, 659–662 (2009).
Borneman, A. R. et al. Divergence of transcription factor binding sites across related yeast species. Science 317, 815–819 (2007).
Man, O. & Pilpel, Y. Differential translation efficiency of orthologous genes is involved in phenotypic divergence of yeast species. Nat. Genet. 39, 415–421 (2007).
Benschop, J. J. et al. A consensus of core protein complex compositions for Saccharomyces cerevisiae. Mol. Cell 38, 916–928 (2010).
Thornton, B. R. et al. An architectural map of the anaphase-promoting complex. Genes Dev. 20, 449–460 (2006).
Meiringer, C. T. A. et al. The Dsl1 protein tethering complex is a resident endoplasmic reticulum complex, which interacts with five soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptors (SNAREs): implications for fusion and fusion regulation. J. Biol. Chem. 286, 25039–25046 (2011).
Trowitzsch, S. et al. Cytoplasmic TAF2–TAF8–TAF10 complex provides evidence for nuclear holo–TFIID assembly from preformed submodules. Nat. Commun. 6, 6011 (2015).
Sun, J. et al. Stn1–Ten1 is an Rpa2–Rpa3–like complex at telomeres. Gene Dev. 23, 2900–2914 (2009).
Zhou, Z. A., He, M. J., Shah, A. A. & Wan, Y. Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div. 11, 9 (2016).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 (2006).
Chang, L., Zhang, Z., Yang, J., McLaughlin, S. H. & Barford, D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature 513, 388–393 (2014).
Zheng, N. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002).
Elde, N. C., Child, S. J., Geballe, A. P. & Malik, H. S. Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature 457, 485–489 (2009).
Bhaya, D., Davison, M. & Barrangou, R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45, 273–297 (2011).
Zachariae, W. & Nasmyth, K. Whose end is destruction: cell division and the anaphase-promoting complex. Gene Dev. 13, 2039–2058 (1999).
Hansen, T. F. & Houle, D. in Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes (eds Pigliucci, M. & Preston, K.) Ch. 6, 130–150 (Oxford Univ. Press, 2004).
Wang, X. & Sommer, R. J. Antagonism of LIN-17/Frizzled and LIN-18/Ryk in nematode vulva induction reveals evolutionary alterations in core developmental pathways. PLoS Biol. 9, e1001110 (2011).
Dalal, C. K. & Johnson, A. D. How transcription circuits explore alternative architectures while maintaining overall circuit output. Gene Dev. 31, 1397–1405 (2017).
Wall, D. P. et al. Functional genomic analysis of the rates of protein evolution. Proc. Natl Acad. Sci. USA 102, 5483–5488 (2005).
Liu, G. W. et al. Gene essentiality is a quantitative property linked to cellular evolvability. Cell 163, 1388–1399 (2015).
Chen, P. P., Wang, D. D., Chen, H., Zhou, Z. Z. & He, X. L. The nonessentiality of essential genes in yeast provides therapeutic insights into a human disease. Genome Res. 26, 1355–1362 (2016).
Dowell, R. D. et al. Genotype to phenotype: a complex problem. Science 328, 469 (2010).
Poelwijk, F. J., Kiviet, D. J., Weinreich, D. M. & Tans, S. J. Empirical fitness landscapes reveal accessible evolutionary paths. Nature 445, 383–386 (2007).
Ostman, B., Hintze, A. & Adami, C. Impact of epistasis and pleiotropy on evolutionary adaptation. Proc. R. Soc. B 279, 247–256 (2012).
Siegal, M. L. & Leu, J. Y. On the nature and evolutionary impact of phenotypic robustness mechanisms. Annu Rev. Ecol. Evol. Syst. 45, 495–517 (2014).
Eme, L., Trilles, A., Moreira, D. & Brochier-Armanet, C. The phylogenomic analysis of the anaphase promoting complex and its targets points to complex and modern-like control of the cell cycle in the last common ancestor of eukaryotes. BMC Evol. Biol. 11, 265 (2011).
Vicente, J. J. & Cande, W. Z. Mad2, Bub3, and Mps1 regulate chromosome segregation and mitotic synchrony in Giardia intestinalis, a binucleate protist lacking an anaphase-promoting complex. Mol. Biol. Cell 25, 2774–2787 (2014).
Wolfe, K. H. & Shields, D. C. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387, 708–713 (1997).
Marcet-Houben, M. & Gabaldon, T. Beyond the whole-genome duplication: phylogenetic evidence for an ancient interspecies hybridization in the baker’s yeast lineage. PLoS Biol. 13, e1002220 (2015).
Byrne, K. P. & Wolfe, K. H. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res 15, 1456–1461 (2005).
Leducq, J. B. et al. Evidence for the robustness of protein complexes to inter-species hybridization. PLoS Genet. 8, e1003161 (2012).
Sangster, T. A., Lindquist, S. & Queitsch, C. Under cover: causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays 26, 348–362 (2004).
Rudashevskaya, E. L., Sickmann, A. & Markoutsa, S. Global profiling of protein complexes: current approaches and their perspective in biomedical research. Expert Rev. Proteomics 13, 951–964 (2016).
Ito, H., Fukuda, Y., Murata, K. & Kimura, A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163–168 (1983).
Schwarzmuller, T. et al. Systematic phenotyping of a large-scale Candida glabrata deletion collection reveals novel antifungal tolerance genes. PLoS Pathog. 10, e1004211 (2014).
Rice, P., Longden, I. & Bleasby, A. EMBOSS: the European molecular biology open software suite. Trends Genet. 16, 276–277 (2000).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 5, 113 (2004).
Zhang, Z. et al. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem. Biophys. Res. Commun. 419, 779–781 (2012).
Wang, D., Zhang, Y., Zhang, Z., Zhu, J. & Yu, J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 8, 77–80 (2010).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Acknowledgements
We thank the members of Leu lab for the helpful discussions and comments on the manuscript. We also thank J. O’Brien for manuscript editing, and K. Swamy and the IMB Bioinformatics Core for the help with bioinformatic analysis. This work was supported by Academia Sinica of Taiwan (grant nos. AS-IA-110-L01 and AS-GCS-110-01) and the National Science and Technology Council of Taiwan (NSTC 111-2326-B-001-015).
Author information
Authors and Affiliations
Contributions
J.-Y.L. conceived the study. H.-Y.L. and J.-Y.L. designed analyses and interpreted results. H.-Y.L., Y.-H.Y. and Y.-T.J. performed the experiments. H.-Y.L. and C.-W.L. performed bioinformatics analysis. H.-Y.L. and J.-Y.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Aashiq Kachroo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Experimental protocol for orthologous gene replacement in S. cerevisiae.
The pRS416–Scer plasmid was transformed into a S. cerevisiae haploid strain JYL1821 before the essential gene was deleted. A pRS413–tet–Scer or pRS413–tet–orthologue plasmid was then transformed into the deletion strain to determine whether it could replace the pRS416–Scer plasmid. If the essential gene could be replaced by its orthologues, the growth rates of the replacement strains were measured.
Extended Data Fig. 2 Distribution of protein sequence conservation and non-synonymous substitution rates in all essential genes and tested candidates.
Fungal orthologues were compared to the corresponding genes in S. cerevisiae. Protein divergence (a) was calculated as one minus protein identity, and Ka (b) represents the non-synonymous substitution rate. A list of 86 candidate genes (see Supplementary Tables 1 and 2) was selected from the 796 S. cerevisiae essential genes with single orthologues in all four tested species. Candidate genes with high and low sequence divergence were slightly overrepresented since we speculated that these two types of genes might reveal specific evolutionary patterns.
Extended Data Fig. 3 Small changes have accumulated in the compatible orthologues.
a, Replacing the endogenous promoter with the Tet-Off promoter does not have a strong impact on the majority of tested genes. The histogram shows the relative growth rates of strains carrying different S. cerevisiae essential genes under the Tet-Off promoter. The strains exhibiting less than 20% difference in growth rate are colored grey. b, Strains carrying distant orthologues are more likely to show different growth rates. Boxplots indicate median (middle line), 25th and 75th percentile (box), and min and max (whiskers) of relative growth rates in the strains carrying orthologues from different species. Different species data were compared to that of the Tet–Scer strains. P values were calculated by paired, two-tailed Student’s t-test. *, P < 0.05. **, P < 0.01. ***, P < 0.005. The relative growth rates in (a) and (b) were obtained by normalizing the growth rate of the Tet promoter strain to the wild-type strain (JYL1821). c, Growth rates of the strains carrying different orthologues. To simplify comparisons, all growth rates were normalized to that of individual Tet–Scer strains. ⃟ Tet–Scer–orthologues; ⃞ Tet–Ncas–orthologues; △ Tet–Klac–orthologues; ▽ Tet–Ylip–orthologues; ◯ Tet–Spom–orthologues. Different species data were compared to that of the Tet–Scer strains. Strains significantly different from Tet–Scer strains (two-tailed Dunnett’s test, P < 0.05) are in red. All strains were grown in rich medium (YPD). Error bars represent the standard error of the mean from three independent colonies (see Supplementary Table 4 for data and detailed statistical information).
Extended Data Fig. 4 The fast-type genes have the lowest protein sequence identity.
Protein identity of each gene was calculated between S. cerevisiae and N. castellii (a) or S. cerevisiae and S. pombe (b), and different groups were compared. Boxplots indicate median (middle line), 25th and 75th percentile (box), and min and max (whiskers). Distributions with different letters (above each boxplot) are significantly different from each other (fast: n = 7, gradual: n = 24, puncutate: n = 7, static: n = 44, two-sided Mann–Whitney U test, P values < 0.05). Source data and detailed statistical information are provided as a source data file.
Extended Data Fig. 5 Divergent residues of Apc11 are located near the interaction interface of Apc2.
The structures of a complete Scer–Apc2 protein and the first 102 residues of Scer–Apc11 were predicted by the Phyre2 server using the human APC/C complex as the template. The predicted structures of Scer–Apc2 and Scer–Apc11 were then aligned to PDB:4UI9 by the PyMOL Molecular Graphics System (see Methods for details). Previous structural studies have shown that only the C-terminal domain of Scer–Apc2 (residues 430–853, in light blue) interacts with Scer–Apc11, and that the N-terminal domain of Scer–Apc2 (residues 1–419, in dark blue) interacts with other APC/C subunits27,33. Scer–Apc11 is colored yellow. The zinc-chelating residues of the canonical RING domain are highlighted in green. The residues chosen for mutagenesis are colored red and numbered.
Extended Data Fig. 6 Cells show growth defects when the chimeric Apc11 proteins are driven by the endogenous Scer–APC11 promoter, suggesting that both the CTD and NTD of Apc2 contribute to the stability of the complex.
All compatibility tests of different proteins were performed in an apc2Δ apc11Δ shuffling strain with plasmids carrying the orthologous or chimeric genes. The growth rates were normalized to that of a control strain (apc2Δ apc11Δ + Scer–APC2 Scer–APC11). NA, data not available due to cell inviability. Error bars represent the standard error of the mean from four independent colonies. Columns sharing the same letters are not significantly different (alpha = 0.05, Tukey-adjusted P values). Source data and detailed statistical information are provided as a source data file.
Extended Data Fig. 7 Alignment of Apc11 sequences from 23 Ascomycota species reveals a few divergent residues in the highly conserved domains.
The fungal sequences of Apc11 were aligned by MUSCLE59. The residues specific to incompatible Ncas–Apc11 and Klac–Apc11 are highlighted in red. The species with tested orthologues are labeled in blue. Residue numbers are based on the Scer–Apc11 sequence.
Supplementary information
Source data
Source Data
Statistical source data for Figs. 1–5 and Extended Data Figs. 1–7.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, HY., Yu, YH., Jhou, YT. et al. Multiple intermolecular interactions facilitate rapid evolution of essential genes. Nat Ecol Evol 7, 745–755 (2023). https://doi.org/10.1038/s41559-023-02029-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02029-5
This article is cited by
-
Altered assembly paths mitigate interference among paralogous complexes
Nature Communications (2024)
-
Differentially used codons among essential genes in bacteria identified by machine learning-based analysis
Molecular Genetics and Genomics (2024)