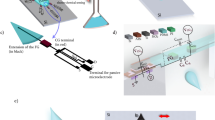
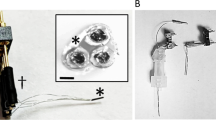
Real-time recording of the kinetics of systemically administered drugs in in vivo microenvironments may accelerate the development of effective medical therapies. However, conventional methods require considerable analyte quantities, have low sampling rates and do not address how drug kinetics correlate with target function over time. Here, we describe the development and application of a drug-sensing system consisting of a glass microelectrode and a microsensor composed of boron-doped diamond with a tip of around 40 μm in diameter. We show that, in the guinea pig cochlea, the system can measure—simultaneously and in real time—changes in the concentration of bumetanide (a diuretic that is ototoxic but applicable to epilepsy treatment) and the endocochlear potential underlying hearing. In the rat brain, we tracked the kinetics of the drug and the local field potentials representing neuronal activity. We also show that the actions of the antiepileptic drug lamotrigine and the anticancer reagent doxorubicin can be monitored in vivo. Our microsensing system offers the potential to detect pharmacological and physiological responses that might otherwise remain undetected.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Li, Y. et al. Sensitive isotope dilution liquid chromatography/tandem mass spectrometry method for quantitative analysis of bumetanide in serum and brain tissue. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 998–1002 (2011).
Patel, D. S. et al. Application of a rapid and sensitive liquid chromatography-tandem mass spectrometry method for determination of bumetanide in human plasma for a bioequivalence study. J. Pharm. Biomed. Anal. 66, 365–370 (2012).
Chaurasia, C. S. et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 24, 1014–1025 (2007).
Bito, L., Davson, H., Levin, E., Murray, M. & Snider, N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J. Neurochem. 13, 1057–1067 (1966).
Massoud, T. F. & Gambhir, S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580 (2003).
Clark, J. J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7, 126–129 (2010).
Wang, J. Electrochemical glucose biosensors. Chem. Rev. 108, 814–825 (2008).
Ivandini, T. A., Rao, T. N., Fujishima, A. & Einaga, Y. Electrochemical oxidation of oxalic acid at highly boron-doped diamond electrodes. Anal. Chem. 78, 3467–3471 (2006).
Fujishima, A., Einaga, Y., Rao, T. N. & Tryk, D. A. Diamond Electrochemistry (Elsevier–BKC, Tokyo, Japan, 2005).
Härtl, A. et al. Protein-modified nanocrystalline diamond thin films for biosensor applications. Nat. Mater. 3, 736–742 (2004).
Koppang, M. D., Witek, M., Blau, J. & Swain, G. M. Electrochemical oxidation of polyamines at diamond thin-film electrodes. Anal. Chem. 71, 1188–1195 (1999).
Yano, T., Tryk, D. A., Hashimoto, K. & Fujishima, A. Electrochemical behavior of highly conductive boron-doped diamond electrodes for oxygen reduction in alkaline solution. J. Electrochem. Soc. 145, 1870–1876 (1998).
Fierro, S. et al. In vivo assessment of cancerous tumors using boron doped diamond microelectrode. Sci. Rep. 2, 901 (2012).
Suzuki, A. et al. Fabrication, characterization, and application of boron-doped diamond microelectrodes for in vivo dopamine detection. Anal. Chem. 79, 8608–8615 (2007).
Nin, F. et al. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl Acad. Sci. USA 105, 1751–1756 (2008).
Green, D. G. & Kellerth, J. O. Postsynaptic versus presynaptic inhibition in antagonistic stretch reflexes. Science 152, 1097–1099 (1966).
Giebisch, G. Measurements of electrical potential differences on single nephrons of the perfused Necturus kidney. J. Gen. Physiol. 44, 659–678 (1961).
Bakan, A., Lazo, J. S., Wipf, P., Brummond, K. M. & Bahar, I. Toward a molecular understanding of the interaction of dual specificity phosphatases with substrates: insights from structure-based modeling and high throughput screening. Curr. Med. Chem. 15, 2536–2544 (2008).
Hibino, H. & Kurachi, Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 21, 336–345 (2006).
Hibino, H., Nin, F., Tsuzuki, C. & Kurachi, Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 459, 521–533 (2010).
Yoshida, T. et al. NKCCs in the fibrocytes of the spiral ligament are silent on the unidirectional K+ transport that controls the electrochemical properties in the mammalian cochlea. Pflugers Arch. 467, 1577–1589 (2015).
Schmiedt, R. A., Lang, H., Okamura, H. O. & Schulte, B. A. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J. Neurosci. 22, 9643–9650 (2002).
Crouch, J. J., Sakaguchi, N., Lytle, C. & Schulte, B. A. Immunohistochemical localization of the Na–K–Cl co-transporter (NKCC1) in the gerbil inner ear. J. Histochem. Cytochem. 45, 773–778 (1997).
Xu, J. C. et al. Molecular cloning and functional expression of the bumetanide-sensitive Na–K–Cl cotransporter. Proc. Natl Acad. Sci. USA 91, 2201–2205 (1994).
Flamenbaum, W. & Friedman, R. Pharmacology, therapeutic efficacy, and adverse effects of bumetanide, a new “loop” diuretic. Pharmacotherapy 2, 213–222 (1982).
Kahle, K. T., Barnett, S. M., Sassower, K. C. & Staley, K. J. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na+–K+–2Cl− cotransporter NKCC1. J. Child Neurol. 24, 572–576 (2009).
Halstenson, C. E. & Matzke, G. R. Bumetanide: a new loop diuretic (Bumex, Roche Laboratories). Drug Intell. Clin. Pharm. 17, 786–797 (1983).
Flamenbaum, W. Diuretic use in the elderly: potential for diuretic-induced hypokalemia. Am. J. Cardiol. 57, 38A–43A (1986).
Rybak, L. P. Ototoxicity of loop diuretics. Otolaryngol. Clin. North Am. 26, 829–844 (1993).
Thorne, M. et al. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope 109, 1661–1668 (1999).
Wangemann, P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 576, 11–21 (2006).
Salt, A. N., Melichar, I. & Thalmann, R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97, 984–991 (1987).
Armbruster, D. A., Tillman, M. D. & Hubbs, L. M. Limit of detection (LQD)/limit of quantitation (LOQ): comparison of the empirical and the statistical methods exemplified with GC–MS assays of abused drugs. Clin. Chem. 40, 1233–1238 (1994).
Kusakari, J., Kambayashi, J., Ise, I. & Kawamoto, K. Reduction of the endocochlear potential by the new “loop” diuretic, bumetanide. Acta Otolaryngol. 86, 336–341 (1978).
Sewell, W. F. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear. Res. 14, 305–314 (1984).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Frelin, C., Chassande, O. & Lazdunski, M. Biochemical characterization of the Na+/K+/Cl− co-transport in chick cardiac cells. Biochem. Biophys. Res. Commun. 134, 326–331 (1986).
Van Mil, H. G. J., Geukes Foppen, R. J. & Siegenbeek van Heukelom, J. The influence of bumetanide on the membrane potential of mouse skeletal muscle cells in isotonic and hypertonic media. Br. J. Pharmacol. 120, 39–44 (1997).
Ben-Ari, Y., Tyzio, R. & Nehlig, A. Excitatory action of GABA on immature neurons is not due to absence of ketone bodies metabolites or other energy substrates. Epilepsia 52, 1544–1558 (2011).
Sipilä, S. T., Schuchmann, S., Voipio, J., Yamada, J. & Kaila, K. The cation–chloride cotransporter NKCC1 promotes sharp waves in the neonatal rat hippocampus. J. Physiol. 573, 765–773 (2006).
Brown, P. D., Davies, S. L., Speake, T. & Millar, I. D. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129, 957–970 (2004).
Gross, J. Analytical methods and experimental approaches for electrophysiological studies of brain oscillations. J. Neurosci. Methods 228, 57–66 (2014).
Lenoir, M., Tang, J. S., Woods, A. S. & Kiyatkin, E. A. Rapid sensitization of physiological, neuronal, and locomotor effects of nicotine: critical role of peripheral drug actions. J. Neurosci. 33, 9937–9949 (2013).
Kim, E. J. & Lee, M. G. Pharmacokinetics and pharmacodynamics of intravenous bumetanide in mutant Nagase analbuminemic rats: importance of globulin binding for the pharmacodynamic effects. Biopharm. Drug Dispos. 22, 147–156 (2001).
Töpfer, M. et al. Consequences of inhibition of bumetanide metabolism in rodents on brain penetration and effects of bumetanide in chronic models of epilepsy. Eur. J. Neurosci. 39, 673–687 (2014).
Walker, M. C., Tong, X., Perry, H., Alavijeh, M. S. & Patsalos, P. N. Comparison of serum, cerebrospinal fluid and brain extracellular fluid pharmacokinetics of lamotrigine. Br. J. Pharmacol. 130, 242–248 (2000).
Hunt, M. J., Garcia, R., Large, C. H. & Kasicki, S. Modulation of high-frequency oscillations associated with NMDA receptor hypofunction in the rodent nucleus accumbens by lamotrigine. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1312–1319 (2008).
Brandt, C., Nozadze, M., Heuchert, N., Rattka, M. & Löscher, W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J. Neurosci. 30, 8602–8612 (2010).
Cleary, R. T. et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS ONE 8, e57148 (2013).
Ward, A. & Heel, R. C. Bumetanide. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs 28, 426–464 (1984).
Lee, S. H., Lee, M. G. & Kim, N. D. Pharmacokinetics and pharmacodynamics of bumetanide after intravenous and oral administration to rats: absorption from various GI segments. J. Pharmacokinet. Biopharm. 22, 1–17 (1994).
Kissinger, P. T., Hart, J. B. & Adams, R. N. Voltammetry in brain tissue—a new neurophysiological measurement. Brain Res. 55, 209–213 (1973).
Robinson, D. L., Venton, B. J., Heien, M. L. & Wightman, R. M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 49, 1763–1773 (2003).
Hashemi, P. et al. Brain dopamine and serotonin differ in regulation and its consequences. Proc. Natl Acad. Sci. USA 109, 11510–11515 (2012).
Liu, J. et al. In vivo electrochemical monitoring of the change of cochlear perilymph ascorbate during salicylate-induced tinnitus. Anal. Chem. 84, 5433–5438 (2012).
Gonon, F., Buda, M., Cespuglio, R., Jouvet, M. & Pujol, J. F. In vivo electrochemical detection of catechols in the neostriatum of anaesthetized rats: dopamine or DOPAC? Nature 286, 902–904 (1980).
Marsden, C. A., Bennett, G. W., Brazell, M., Sharp, T. & Stolz, J. F. Electrochemical monitoring of 5-hydroxytryptamine release in vitro and related in vivo measurements of indoleamines. J. Physiol. Paris 77, 333–337 (1981).
Nakazato, T. & Akiyama, A. In vivo electrochemical measurement of the long-lasting release of dopamine and serotonin induced by intrastriatal kainic acid. J. Neurochem. 69, 2039–2047 (1997).
Sceniak, M. P. & Maciver, M. B. Cellular actions of urethane on rat visual cortical neurons in vitro. J. Neurophysiol. 95, 3865–3874 (2006).
Pagliardini, S., Funk, G. D. & Dickson, C. T. Breathing and brain state: urethane anesthesia as a model for natural sleep. Respir. Physiol. Neurobiol. 188, 324–332 (2013).
Gad, S. C. & Gad, S. C. Safety Pharmacology in Pharmaceutical Development: Approval and Post Marketing Surveillance 2nd edn (CRC Press, Boca Raton, FL, 2012).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Hibino, H. et al. An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4.1), in cochlear stria vascularis of inner ear: its specific subcellular localization and correlation with the formation of endocochlear potential. J. Neurosci. 17, 4711–4721 (1997).
Natsume, K., Hallworth, N. E., Szgatti, T. L. & Bland, B. H. Hippocampal theta-related cellular activity in the superior colliculus of the urethane-anesthetized rat. Hippocampus 9, 500–509 (1999).
Acknowledgements
We thank P. Bredeloux for comments on the experimental results, and Y. Takahashi and H. Shiku for technical advice. This study was partially supported by the following research grants: Grant-in-Aid for Scientific Research B 25293058 (to H.H.); Grant-in-Aid for Scientific Research C 15K10770 (to K.D.); and Grants-in-Aid for Young Scientists B 25870248 (to F.N.) and 26870210 (to G.O.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and JST-ACCEL (to Y.E.). In addition, funds were provided by the Nakatani Foundation (to H.H.), Takeda Science Foundation (to F.N.), Uehara Memorial Foundation (to F.N.) and Astellas Foundation for Research on Metabolic Disorders (to F.N.).
Author information
Authors and Affiliations
Contributions
G.O., F.N., H.K., T.Y., Y.E. and H.H. designed the experiments. G.O. and K.D. developed the experimental setup. F.N., T.Y. and S.K. were involved in developing the surgical procedures. Y.I., K.A. and Y.E. prepared the BDD electrodes. Y.I., K.A., Y.E. and M.T. contributed to establishing the protocol for the electrochemical experiments. G.O. performed the electrochemical experiments. T.H., T.O. and K.H. supported the data collection. Y.S., K.M. and H.K designed the LC–MS/MS experiments. Y.S. performed the LC–MS/MS experiments. G.O., S.S., T.O. and I.F. analysed the results. G.O., T.O., I.F. and H.H. wrote the paper. All authors edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary figures, tables and references
Rights and permissions
About this article
Cite this article
Ogata, G., Ishii, Y., Asai, K. et al. A microsensing system for the in vivo real-time detection of local drug kinetics. Nat Biomed Eng 1, 654–666 (2017). https://doi.org/10.1038/s41551-017-0118-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-017-0118-5
This article is cited by
-
2D MOF-enhanced SPR detector based on tunable supramolecular probes for direct and sensitive detection of DOX in serum
Microchimica Acta (2024)
-
An ultra-small bispecific protein augments tumor penetration and treatment for pancreatic cancer
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo
Analytical and Bioanalytical Chemistry (2021)
-
Characterisation of the static offset in the travelling wave in the cochlear basal turn
Pflügers Archiv - European Journal of Physiology (2020)
-
Real-time drug pharmacokinetics
Nature Biomedical Engineering (2017)