Abstract
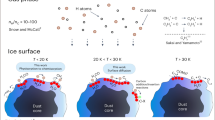
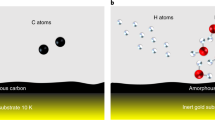
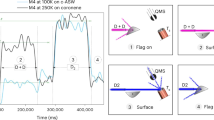
In molecular clouds at temperatures as low as 10 K, all species except hydrogen and helium should be locked in the heterogeneous ice on dust grain surfaces. Nevertheless, astronomical observations have detected over 150 different species in the gas phase in these clouds. The mechanism by which molecules are released from the dust surface below thermal desorption temperatures to be detectable in the gas phase is crucial for understanding the chemical evolution in such cold clouds. Chemical desorption, caused by the excess energy of an exothermic reaction, was first proposed as a key molecular release mechanism almost 50 years ago1. Chemical desorption can, in principle, take place at any temperature, even below the thermal desorption temperature. Therefore, astrochemical network models commonly include this process2,3. Although there have been a few previous experimental efforts4,5,6, no infrared measurement of the surface (which has a strong advantage to quantify chemical desorption) has been performed. Here, we report the first infrared in situ measurement of chemical desorption during the reactions H + H2S → HS + H2 (reaction 1) and HS + H → H2S (reaction 2), which are key to interstellar sulphur chemistry2,3. The present study clearly demonstrates that chemical desorption is a more efficient process for releasing H2S into the gas phase than was previously believed. The obtained effective cross-section for chemical desorption indicates that the chemical desorption rate exceeds the photodesorption rate in typical interstellar environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Williams, D. A. Physical adsorption processes on interstellar graphite grains. Astrophys. J. 151, 935–943 (1968).
Garrod, R. T., Wakelam, V. & Herbst, E. Non-thermal desorption from interstellar dust grains via exothermic surface reactions. Astron. Astrophys. 467, 1103–1115 (2007).
Vidal, T. H. G. et al. On the reservoir of sulphur in dark clouds: chemistry and elemental abundance reconciled. Mon. Not. R. Astron. Soc. 489, 435–447 (2017).
Dulieu, F. et al. How micron-sized dust particles determine the chemistry of our Universe. Sci. Rep. 3, 1338 (2013).
Minissale, M., Dulieu, F., Cazaux, S. & Hocuk, S. Dust as interstellar catalyst. I. Quantifying the chemical desorption process. Astron. Astrophys. 585, A24 (2016).
He, J., Emtiza, S. M. & Vidali, G. Mechanism of atomic hydrogen addition reactions on np-ASW. Astrophys. J. 851, 104 (2017).
Tielens, A. G. G. M. The molecular universe. Rev. Mod. Phys. 85, 1021–1081 (2013).
Hama, T. & Watanabe, N. Surface processes on interstellar amorphous solid water: adsorption, diffusion, tunneling reactions, and nuclear-spin conversion. Chem. Rev. 113, 8783–8839 (2013).
van Dishoeck, E. F., Herbst, E. & Neufeld, D. A. Interstellar water chemistry: from laboratory to observations. Chem. Rev. 113, 9043–9085 (2013).
Hama, T. et al. A desorption mechanism of water following vacuum-ultraviolet irradiation on amorphous solid water at 90 K. J. Chem. Phys. 132, 164508 (2010).
Fayolle, E. C. et al. CO ice photodesorption: a wavelength-dependent study. Astrophys. J. Lett. 739, L36 (2011).
Muñoz Caro, G. M. et al. New results on thermal and photodesorption of CO ice using the novel InterStellar Astrochemistry Chamber (ISAC). Astron. Astrophys. 522, A108 (2010).
Cuppen, H. H. et al. Grain surface models and data for astrochemistry. Space Sci. Rev. 212, 1–58 (2017).
Watanabe, N., Nagaoka, A., Shiraki, T. & Kouchi, A. Hydrogenation of CO on pure solid CO and CO–H2O mixed ice. Astrophys. J. 616, 638–642 (2004).
Hidaka, H., Miyauchi, N., Kouchi, A. & Watanabe, N. Structural effects of ice grain surfaces on the hydrogenation of CO at low temperatures. Chem. Phys. Lett. 456, 36–40 (2008).
Lamberts, T. & Kästner, J. Tunneling reaction kinetics for the hydrogen abstraction reaction H + H2S → H2 + HS in the interstellar medium. J. Phys. Chem. A 121, 9736–9741 (2017).
Qi, J., Lu, D., Song, H., Li, J. & Yang, M. Quantum and quasiclassical dynamics of the multi-channel H + H2S reaction. J. Chem. Phys. 146, 124303 (2017).
Fathe, K., Holt, J. S., Oxley, S. P. & Pursell, C. J. Infrared spectroscopy of solid hydrogen sulfide and deuterium sulfide. J. Phys. Chem. A 110, 10793–10798 (2006).
Jiménez-Escobar, A. & Muñoz Caro, G. M. Sulfur depletion in dense clouds and circumstellar regions. I. H2S ice abundance and UV-photochemical reactions in the H2O-matrix. Astron. Astrophys. 536, A91 (2011).
Collings, M. P. et al. A laboratory survey of the thermal desorption of astrophysically relevant molecules. Mon. Not. R. Astron. Soc. 354, 1133–1140 (2004).
Watanabe, N. et al. Direct measurements of hydrogen atom diffusion and the spin temperature of nascent H2 molecule on amorphous solid water. Astrophys. J. Lett. 714, L233–L237 (2010).
van der Tak, F. F. S., Boonmanm, A. M. S., Braakman, R. & van Dishoeck, E. F. Sulphur chemistry in the envelopes of massive young stars. Astron. Astrophys. 412, 133–145 (2003).
Woods, P. M. et al. A new study of an old sink of sulphur in hot molecular cores: the sulphur residue. Mon. Not. R. Astron. Soc. 450, 1256–1267 (2015).
Taquet, V., Charnley, S. B. & Sipilä, O. Multilayer formation and evaporatin of deuterated ices in prestellar and protostellar cores. Astrophys. J. 791, 1 (2014).
Calmonte, U. et al. Sulphur-bearing species in the coma of comet 67P/Churyumov–Gerasimenko. Mon. Not. R. Astron. Soc. 462, S253–S273 (2016).
Esplugues, G. B., Viti, S., Goicoechea, J. R. & Cernicharo, J. Modelling the sulphur chemistry evolution in Orion KL. Astron. Astrophys. 567, A95 (2014).
Holdship, J. et al. H2S in the L1157-B1 bow shock. Mon. Not. R. Astron. Soc. 463, 802–810 (2016).
Cruz-Diaz, G. A., Muñoz Caro, G. M., Chen, Y.-J. & Yih, T.-S. Vacuum-UV spectroscopy of interstellar ice analogs I. Absorption cross-sections of polar-ice molecules. Astron. Astrophys. 562, A119 (2014).
Prasad, S. S. & Tarafdar, S. P. UV radication field inside dense clouds: its possible existence and chemical implications. Astrophys. J. 267, 603–609 (1983).
Shen, C. J., Greenberg, J. M., Schutte, W. A. & van Dishoeck, E. F. Cosmic ray induced explosive chemical desorption in dense clouds. Astron. Astrophys. 415, 203–215 (2004).
Gerakines, P. A., Schutte, W. A., Greenberg, J. M. & van Dishoeck, E. F. The infrared band strengths of H2O, CO and CO2 in laboratory simulations of astrophysical ice mixtures. Astron. Astrophys. 296, 810–818 (1995).
Hama, T. et al. The mechanism of surface diffusion of H and D atoms on amorphous solid water: existence of various potential sites. Astrophys. J. 757, 185 (2012).
Miyauchi, N. et al. Formation of hydrogen peroxide and water from the reaction of cold hydrogen atoms with solid oxygen at 10 K. Chem. Phys. Lett. 456, 27–30 (2008).
Oba, Y., Osaka, K., Watanabe, N., Chigai, T. & Kouchi, A. Reaction kinetics and isotope effect of water formation by the surface reaction of solid H2O2 with H atoms at low temperatures. Faraday Discuss. 168, 185–204 (2014).
Minissale, M. & Dulieu, F. Influence of surface coverage on the chemical desorption process. J. Chem. Phys. 141, 014304 (2014).
Minissale, M., Moudens, A., Baouche, S., Chaabouni, H. & Dulieu, F. Hydrogenation of CO-bearing species on grains: unexpected chemical desorption of CO. Mon. Not. R. Astron. Soc. 458, 2953–2961 (2016).
Watanabe, N. & Kouchi, A. Ice surface reactions: a key to chemical evolution in space. Prog. Surf. Sci. 83, 439–489 (2008).
Zhao, Y. & Truhlar, D. G. Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J. Chem. Phys. A 108, 6908–6918 (2004).
Weigend, F., Häser, M., Patzelt, H. & Ahlrichs, R. RI-MP2: optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 294, 143–152 (1998).
Frisch, M. J. et al. Gaussian 09 Revision D.01 (Gaussian, Wallingford, CT, 2016).
Acknowledgements
The authors thank H. Hidaka, T. Hama and J. Kästner for discussions about chemical desorption. This work was partly supported by a Japan Society for the Promotion of Science Grant-in-Aid for Specially Promoted Research (JP17H06087) and Grant-in-Aid for Young Scientists (A) (JP26707030). Computational resources were provided by the state of Baden-Württemberg through bwHPC and the German Research Foundation through grant number INST 40/467-1 FUGG.
Author information
Authors and Affiliations
Contributions
Y.O. planned the experiments in consultation with N.W. and A.K. Y.O. and T.T. performed the experiments. T.L. performed the computational calculations on binding energy. All authors discussed the results. Y.O., T.L. and N.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–3, Supplementary Table 1
Rights and permissions
About this article
Cite this article
Oba, Y., Tomaru, T., Lamberts, T. et al. An infrared measurement of chemical desorption from interstellar ice analogues. Nat Astron 2, 228–232 (2018). https://doi.org/10.1038/s41550-018-0380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41550-018-0380-9
This article is cited by
-
Unraveling sulfur chemistry in interstellar carbon oxide ices
Nature Communications (2022)
-
Rotational and nuclear-spin level dependent photodissociation dynamics of H2S
Nature Communications (2021)
-
Ultraviolet photolysis of H2S and its implications for SH radical production in the interstellar medium
Nature Communications (2020)