Abstract
The health burden of Parkinson’s disease (PD) and diabetes increases rapidly in China. However, no population-based study of the association between glucose lowering agents and PD incidence has been conducted in mainland Chinese population. Preclinical studies indicate that thiazolidinediones (TZDs) have neuroprotective effects against PD through stimulating peroxisome proliferator-activated receptor gamma. Nevertheless, debate remains in human studies. We assembled a retrospective cohort of type 2 diabetes mellitus (T2DM) patients who were new users of TZDs or alpha glucosidase inhibitors (AGIs) using the Yinzhou Regional Health Care Database. A Cox model with inverse probability of treatment weighting (IPTW) was applied to estimate the hazard ratio (HR) of PD incidence associated with the use of TZDs compared with AGIs. The final cohort included 12,704 new users of TZDs and 49,696 new users of AGIs. The incidence of PD was 135 per 100,000 person-years in TZD users and 203 per 100,000 person-years in the AGI group. An inverse association between use of TZDs and incidence of PD, with a HR of 0.74 (95% confidence interval, 0.59–0.92), was observed after adjusting for potential confounding using IPTW. The results of various subgroup analyses and sensitivity analyses were consistent with the findings of the primary analysis. Our results indicated that the use of TZD is associated with a decreased risk of PD incidence in a mainland Chinese population with T2DM. Given the heavy disease burden of PD and diabetes in China, these findings could provide some evidence for developing effective prevention and control measures to reduce the future incidence of PD in China.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease, characterized by motor features such as bradykinesia, rigidity, tremors, and postural instability1. This disorder contributes millions of disability adjusted life-years to the global burden of disease and is associated with a variety of risk factors1,2, among which type 2 diabetes mellitus (T2DM) is found to increase the risk of PD in a quite number of clinical studies3,4. This appears to be an important finding in the context of a rapidly increasing disease burden of diabetes and PD1,5. As these two diseases share several overlapping mechanisms such as oxidative stress, aberrant protein accumulation, mitochondrial dysfunction, and chronic inflammation3,4, much attention has been paid to the potential relationship between glucose lowering agents and neurodegeneration and whether these drugs could have some effects in preventing or treating PD3,6,7.
Thiazolidinediones (TZDs) are a class of important second-line glucose lowering agents, improving glycemic control in T2DM patients by increasing insulin sensitivity3,7. A variety of preclinical studies have suggested a neuroprotective effect of TZDs in PD models3,4, motivating several epidemiological studies of the association between TZD use and risk of PD incidence. Nevertheless, results of these studies are conflicting due to methodology heterogeneity, population difference, and various follow-up periods8,9. Furthermore, most of these studies have been conducted in Western countries, while only several similar studies of the association between TZD use and PD risk were reported in the Asia, where more than 60% of the world’s population aged over 65 years live10. Although, China has the highest disease burden and fastest growth of T2DM and PD1,5, no study has been performed to assess the effects of TZD use on PD risk in a mainland Chinese population. To fill this gap, we conducted a retrospective cohort study using a well-established electronic healthcare database in China to evaluate the association between TZD use and risk of PD in patients with T2DM.
Results
Basic characteristics
We included 62,400 T2DM patients in the final cohort. Of these, 49,696 participants initiated alpha glucosidase inhibitors (AGIs) and 12,704 were new users of TZDs (Fig. 1). The median follow-up time for new users of AGIs and TZDs was 5.3 (interquartile range [IQR], 2.5–8.7; maximum 13) and 6.1 (IQR, 3.1–8.8; maximum 13) years, respectively. Patients’ characteristics at baseline are given in the Table 1. Compared with AGI new users, there was a lower proportion of participants older than 60 years or having higher education in TZD users. Further, new users of TZDs were less likely to be hospitalized, have a Charlson comorbidity index (CCI) > 1, and receive insulin treatment in the baseline period. However, participants in the TZD group had higher body mass index (BMI) and were more likely to receive outpatient care and prescriptions of metformin and sulfonylureas at baseline. Nevertheless, after weighted by inverse probability of treatment, all baseline characteristics were effectively balanced between the exposure and comparison group (standardized mean difference [SMD] < 0.1 for all covariates, Table 1).
Association between TZD use and PD incidence
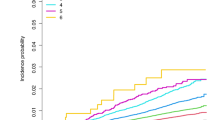
A total of 674 incident cases of PD were observed during follow-up in the primary analysis, of them 571 were AGI users and 103 were TZD users, with an incidence of 203 and 135 per 100,000 person-years, respectively. Compared with users of AGIs, the crude analysis indicated a significant inverse association between use of TZDs and incidence of PD, with a hazard ratio (HR) of 0.67 (95% confidence interval [CI], 0.54–0.82) (Table 2). After adjusting for potential confounders using stabilized inverse probability of treatment weighting (IPTW), users of TZDs still showed a significant 26% decrease in incidence of PD (HR 0.74; 95% CI, 0.59–0.92). The mean of the IPTW was 1.00 (standard deviation, 0.24), and the median was 0.97 (IQR, 0.50–1.05). Multivariate regression model presented consistent result, with a HR of 0.76 (95% CI, 0.62–0.95). Figure 2 gives the IPTW-weighted survival curves of the users of AGIs and TZDs. Moreover, subgroup analyses found similar inverse association between use of TZDs and incidence of PD in different subpopulations of T2DM patients except that in smokers TZD use showed an insignificant increase of risk of PD (HR 1.02; 95% CI, 0.64–1.64). No significant interaction was indicated in all subgroup analyses (Table 2). In addition, the IPTW adjusted HRs for the cumulative duration of TZD use of ≤0.5 years, 0.51–4.0 years, and >4 years were 0.80 (95% CI, 0.51–1.26), 0.69 (95% CI, 0.45–1.07), and 0.73 (95% CI, 0.55–0.98), respectively (Supplementary Table 1).
Sensitivity analyses
Table 3 shows the results of per-protocol (PP) analysis and all sensitivity analyses. In the PP analysis, participants were censored when ceasing the initial drug, resulting in a mean follow-up of 2.2 years. Crude and IPTW analyses showed a HR of 0.65 (95% CI, 0.44–0.95) and 0.81 (95% CI, 0.53–1.21), respectively. When further adjusting for potential selection bias due to artificial censoring using inverse probability of censoring weighting (IPCW), the HR changed to 0.73 (95% CI, 0.45–1.20).
All sensitivity analyses were consistent with that of the primary analysis (Table 3). First, analysis using a washout period of 1 year indicated that the HR of TZD use was 0.76 (95% CI, 0.60–0.95). Further results of washout periods of 18 and 24 months were consistent. Then the second sensitivity analysis got a HR of 0.74 (95% CI, 0.59–0.93) after excluding five cases of potential secondary PD. Third, when the IPTW was not truncated, analysis presented a HR of 0.78 (95% CI, 0.61–1.00), indicating extreme weights might bias the result to the null value. In contrast, result of the unstabilized IPTW model was the same as the primary analysis. Fourth, competing risk due to all-cause mortality did not appear to an issue as the subdistribution hazard model yielded a HR of 0.74 (95% CI, 0.60–0.91), almost the same as that in the primary analysis. Fifth, when we restricted the population to T2DM patients aged 40 years or older, a HR of 0.74 (95% CI, 0.59–0.93) was observed. Sixth, results of different definitions of incident PD were consistent, with HRs ranged from 0.74 to 0.77. Seventh, in the subcohort of participants who initiated monotony therapy of TZDs, we got a HR of 0.73 (95% CI, 0.53–1.00) compared with monotony treatment of AGIs. Finally, excluding potential latency periods of different length resulted in similar HR estimates, all of which were significant lower than 1, ranging from 0.67 (95% CI, 0.51–0.88) to 0.74 (95% CI, 0.58–0.94) (Fig. 3).
Discussion
In this long-term population-based cohort study, participants were followed-up for a maximum of approximating 13 years and we found that use of TZDs was associated with a 26% decrease in the incidence of PD compared with AGI use in patients with T2DM. Results of an emulation of PP effects in which follow-up was censored when participants discontinuing the initial treatment, showed a HR in the same direction and of similar magnitude as those observed in primary analyses. Further, our results were consistent in various subgroup analyses and robust across different sensitivity analyses.
To the best of our knowledge, no previous cohort study has investigated the association between TZD use and risk of PD in a mainland Chinese population. PD is the fastest growing neurological disorder1,10, causing a substantial amount of disease burden worldwide. This disease is a particular concern in China, where an estimate of 3.6 million people aged 60 years or above live with PD11, accounting for 23% of the entire global PD population1,12. Further, China has the fastest increasing in prevalence of PD, which was more than five folds that of the global average10,12. It is estimated that the number of PD patients in China will increase to around five million by 2030, accounting for 60% of patients with PD of the whole world10. Therefore, our findings of the potential neuroprotective effect of TZDs in T2DM patients might provide some insights into the development of effective prevention and control measures to reduce the future burden of PD10,11.
Our findings were consistent with previous studies in vivo and in vitro. PD pathogenesis involves multiple mechanisms, among which in vivo and in vitro studies have shown that TZDs may play a role in improving mitochondrial function and reducing insulin resistance, oxidative stress, and inflammation4,13. TZDs are a kind of agonist of peroxisome proliferator-activated receptor γ (PPARγ), reducing insulin resistance through regulating genes involving insulin sensitivity4,7. They can also bind to the outer mitochondrial membrane protein (mitoNEET), which plays a key role in electron transport and oxidative phosphorylation, to regulate neuronal complex I activity in neuronal cells, reducing oxidative stress and cell death4,7. Moreover, animal experiments have shown that pioglitazone is able to protect against lipopolysaccharide-mediated inflammation and dopaminergic neurodegeneration in the rat brain14. Also, TZDs present neuroprotective effects in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated mice through influencing attenuation of microglial response, activation of kappa-light-chain-enhancer of activated B-cells (NF-κB) and production of inflammatory cytokines15,16.
Despite showing promise in multiple researches of the potential mechanisms through which TZDs may have effects on PD, only a limited number of clinical studies have investigated the association between TZD use and PD incidence in diabetic patients. Among these, the findings of this study were consistent with several previous studies conducted in the United Kingdom17, Norway18, and Taiwan region19,20, of which all had a follow-up time of more than 5 years, and reported a significant reduction in the incidence of PD in TZD users, with a HR ranging from 0.40 to 0.72. No association between TZD use and risk of PD was observed in other studies6,18,21. However, all of these studies followed participants for an average of less than 5 years, which may not long enough to observe the protective effect of TZDs8,9, since PD has a long onset period22. This was also reflected in our PP analysis, in which participants were censored at cessation of initial treatment and thus resulted in an average of 2 years of follow-up. The PP analysis observed an insignificant but similar magnitude of reduction in PD incidence in TZD users as that in primary analysis. In addition, a case-control study in Finland reported that TZD use might reduce PD risk by 22%22, approximating our results. Furthermore, several methodological issues should be noted in previous studies. Various comparison groups have been used in previous studies, among which the majority used non-users of TZDs as the control19,20,21,23,24. Some studies even included patients who did not receive any glucose lowering drugs19,20,21,23. This could induce several biases in the results, including confounding by indication and immortal time bias25,26. Since TZDs are usually prescribed as a second-line drug, studies included patients only receiving treatment of metformin which is the first-line choice for glycemic control in the comparison group18,19,20,21,23, had a risk of time-lag bias26. Another limitation that most studies had was the uncontrolled confounding by lifestyle factors, such as smoking and drinking status, and disease severity of diabetes, which has been shown to be associated with the risk of PD2,8.
In this study we applied an active-comparator new-user (ACNU) design using AGIs which were another class of commonly used second-line hypoglycemic drugs in the study population27 as the comparator, with a rich set of potential confounders, including lifestyle factors and disease severity measures, thus mitigating time-related bias and confounding by indication25,28. However, despite these epidemiological studies, clinical trials investigating effects of TZDs on PD risk are scarce. A phase 2 randomized controlled trial concluded that pioglitazone had no effects on modifying progression in early PD29. Nevertheless, this trial only included early PD patients without diabetes. Therefore, the negative findings might be due to the exclusion of patients with diabetes, as diabetes and PD share some common pathogenesis9. Therefore, TZDs may only have a neuroprotective effect in diabetes patients, but not in nondiabetes population8,9. Furthermore, a short follow-up of 44 weeks in this trial might not be sufficient to observe a significant protective effect with clinical value8,9. In contrast, our study was aimed to assess the association between TZD use and PD incidence and could not determine whether this drug could modify the progression of PD after disease onset. Furthermore, our results did not indicate any significant effect modification by other risk factors in the association between TZD use and PD risk. This was consistent with several previous studies, which also found no interaction between TZD use and age or sex6,21. However, potential heterogeneity of treatment effects should be noted in some population, as our results indicated that TZD use might have a stronger effect in T2DM patients under 60 years of age. Further studies with larger sample size are need for investigating potential heterogeneous effects of TZD use on PD risk.
Several limitations should be noted in this study. First, participants in this study were from a single municipal district in China, thus caution should be exercised when extrapolating the findings of this study to other populations. Second, although we considered quite a number of time-invariant and time-varying covariates in the analyses and ACNU design might have further mitigated the bias related to unmeasured confounding25, some potential confounders, such as dietary patterns, were not adjusted due to lack of accurate information in the database. Third, the database did not record complete dosage information of drugs, making it difficult to study the relationship between cumulative TZD exposure and PD incidence. Our analyses of cumulative duration of TZDs might not accurately reflect the cumulative exposure dose of this drug, thus more studies focusing on the dose-response relationship between cumulative TZD exposure and risk of PD would provide further valuable evidence. Finally, like previous studies6,18,19,20,21,24 we used a by-proxy definition of PD. The PD incidence in our primary analysis was higher than that in diabetic patients in Western countries6,18,24, but similar to that in the Korea23. To adjust for potential bias caused by misclassification of PD cases, we applied several different outcome definitions combing diagnosis and prescription records related to PD. Further, we excluded potential secondary PD. Sensitivity analyses of various PD definitions were highly consistent, thus suggesting that there might be little differences in misclassification of outcome between users of AGIs and TZDs and our results were robust against potential misclassification bias.
In conclusion, we found a potential protective effect of TZD use against PD incidence in a Chinese T2DM population. Given the heavy disease burden of diabetes and PD in China, our results can provide some evidence for selection of oral glucose lowering agents for T2DM in clinical practice.
Methods
Data source
Participants were drawn from the Yinzhou Regional Health Care Database (YRHCD), which integrated longitudinal information of electronic medical records, disease registry and management, death registry and other healthcare services in the Yinzhou District, Ningbo City of China27,30. In 2008, disease registry and management systems were established for diabetes mellitus, cancer, cardiovascular disease, hypertension, and chronic obstructive pulmonary disease. Diabetes patients were registered in the disease surveillance system once diagnosed and would be followed up at least four times a year by community physicians, with common health measures, including blood pressure, fasting plasma glucose (FPG), glycated hemoglobin (HbA1c) being measured or asked27. In this study, disease registry system and electronic diagnosis records were linked and patients with T2DM were included if they: (1) were registered in the diabetes registry system and diagnosed with T2DM; or (2) had more than two diagnosis records of T2DM and no records of type 1 diabetes in the electronic medical records. Longitudinal records of drug prescription, laboratory examination, and outpatient and inpatient visits were linked for information of drug exposure, covariates, and outcome capture. The data used in this study and their relationship were presented in the Supplementary Fig. 1.
Exposure and cohort
We applied an ACNU design, using a comparator of alpha glucosidase inhibitors (AGIs), which are another class of second-line oral glucose lowering agents commonly used at the same stage of T2DM as TZDs in China31. Cohort of T2DM patients who were new users of TZDs or AGIs after January 1, 2009 were assembled. Drug prescriptions were identified by classification code of Anatomical Therapeutic Chemical (ATC) system. TZDs (ATC A10BG) used in the study population contained pioglitazone and rosiglitazone, and AGIs (ATC A10BF) included acarbose, miglitol, and voglibose (Supplementary Table 2). New users were identified by using a baseline washout period of 6 months before the first fill of TZDs or AGIs, during which participants could not receive prescription of a drug from either class. The date of the first fill was defined as the index date.
We excluded participants who were younger than 18 years and who initiated combination treatment of TZDs and AGIs at the index date, and who had received a diagnosis of PD before the index date. We further excluded patients who did not take at least two consecutive prescriptions of TZDs or AGIs within 6 months of the index date to ensure that participants actually started on these drugs.
The study was approved by the ethical review board of Peking University Health Science Center (approval number: IRB00001052-18013-Exempt). Informed consent was not required owing to the use of anonymized routine data.
Outcome and follow-up
The primary outcome was the incident diagnosis of PD, which was defined as (1) having at least two consecutive diagnosis code or description of G20 from the International Classification of Diseases, 10th Revision (ICD-10), or (2) having at least one diagnosis record of PD and prescription records of anti-Parkinson agents (ATC N04, Supplementary Table 3). The date of the first diagnosis was defined as the outcome date.
Our primary analysis was to assess the any-exposure intention-to-treat (ITT) effects of TZD use on the risk of PD. Thus, participants were followed-up from the index date until the first occurrence of the following events: diagnosis of PD, death, last medical record in the database, or the end of the study period (December 31, 2021).
Covariates
Covariates were measured in the baseline washout period and included demographic characteristics (age, sex, and education level); behavior and lifestyle (smoking, drinking, and regular exercise); duration of T2DM; comorbidities measured as CCI (Supplementary Table 4)32; co-use of prescription drugs, including other antidiabetic drugs except TZDs and AGIs (insulins, metformin, sulfonylureas, glinides, and other oral glucose lowering agents), common medications for cardiovascular diseases (diuretics, beta-blocking agents, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and aspirin), lipid modifying agents, and proton-pump inhibitors (PPI). Further, blood glucose level (FPG and HbA1c), blood lipid level, blood pressure, body mass index (BMI), and healthcare utilization (inpatient and outpatient visits) were also included.
Statistical analyses
Descriptive statistics (mean and standard deviation for continuous covariates and frequency and percentage for categorical variables) summarized baseline covariates and standardized mean difference (SMD) was used for comparisons between initiators of TZDs and AGIs as suggested by Austin et al.33. A SMD less than 0.1 was used to show comparable balance in the covariates33. Inverse probability of treatment weighting (IPTW) was applied for controlling baseline confounding and a Cox regression model was used to estimate the HR with 95% confidence interval (CI) for the association between TZD use and PD. We fitted a logistic regression model to estimate the stabilized IPTW for each subject. The denominator of the IPTW was the probability of receiving the actual drug treatment condition on all measured covariates listed above. Continuous variables such as FPG, HbA1c were modeled as a restricted cubic spline with knots at 5th, 25th, 50th, 75th, and 95th percentiles. The numerator of the IPTW was the marginal probability of TZD or AGI use in the overall sample. We also included the year of index date in the model for IPTW to adjust for changes in prescribing patterns over time. The final stabilized IPTW was truncated at the 1st/99th percentile for mitigating impacts of extreme weights. Another two standard Cox models with different confounding adjustment strategies were provided for comparison: unadjusted and multivariate regression adjusted. Multiple imputation was applied for imputing missing data using the full conditional specification method with five imputations according to the quadratic rule recommended by von Hippel34. The proportional hazards assumption was tested using the Schoenfeld residuals method and no violation of this assumption was found. Robust variance was used for calculating the 95% CIs of HRs when applying weighted models.
Subgroup analyses and cumulative duration of TZD use
We examined the association of TZD use and incidence of PD within different subgroups for checking potential interactions between the TZD use and baseline characteristics: age (≤60 and >60 years), sex (female and male), CCI (0 and ≥1), smoking and drinking behavior, FPG (≤7 mmol/L and >7 mmol/L), HbA1c (≤7% and >7%), BMI (≤24 kg/m2 and >24 kg/m2), and duration of diabetes at the index date (≤2.5 years and >2.5 years).
Cumulative duration of TZD use was calculated as the period between the index date and the date of the last TZD prescription. Tertiles of cumulative duration were used to define a categorical cumulative TZD therapy. We then examined the association between different duration (≤0.5, 0.51–4, and >4 years) of TZD treatment and PD incidence compared with use of AGIs.
Sensitivity analyses
We emulated a per-protocol (PP) analysis to examine the effects of sustained exposure, in which participants were further censored upon discontinuation of the initial drug in addition to the censoring reasons in the ITT analyses. Treatment discontinuation was defined as no further refill of the initial drug within 6 months of the previous prescription. This artificial censoring could induce selection bias and could be influenced by time-varying confounders35. Thus, we applied a marginal structural model with time-varying inverse probability of censoring weighting (IPCW) to adjust for selection bias introduced by artificial censoring. IPCW was the inverse of the probability of remaining uncensored at each follow-up conditioned on time-invariant and time-varying factors, and was estimated using a pooled logistic model. Time-varying factors were all covariates listed above except demographic characteristics, behavior and lifestyle, duration of T2DM, and year of index date. We used a 6-month interval for assessing time-updated exposure and covariates at the beginning of each new period in the PP analysis.
We further performed multiple sensitivity analyses to examine the robustness of the results in our primary analysis. First, alternative washout periods of 12, 18, and 24 months were applied for defining new users of TZDs and AGIs. Second, we excluded all possible cases of secondary PD, who had a diagnosis of ICD-10 code G21 or G22 after receiving first diagnosis of G20. Third, unstabilized and untruncated stabilized IPTW were applied. Forth, the Fine-Gray subdistribution hazard model was used to check possible competing risk by death from any cause. Fifth, we restricted the study population to T2DM patients who aged over 40 years at the index date. This could help exclude cases of early-onset PD since young people rarely develop this disease. Sixth, we used several alternative definitions of incident PD as: (1) having at least two diagnoses of G20; (2) having over two diagnoses of G20 and prescriptions of anti-Parkinson agents (ATC N04) after the first diagnosis; and (3) having a consecutive diagnosis of G20 or prescription of anti-Parkinson agents within one year of the first diagnosis of PD. Seventh, we excluded T2DM patients who received any antidiabetic drugs in the baseline washout period and evaluated the effects of monotony therapy of TZDs compared with monotony treatment of AGIs. Finally, we excluded a period after the index date for adjusting potential latency time. For example, we identified participants with over 6 months of use of the initial drug and follow-up were begun 6 months after the index date. This analysis was repeated with requirements of successively longer minim time of follow-up by adding 1 month to each analysis, up to a maximum of 24 months. This kind of analysis could also help adjust for unmeasured confounding by undiagnosed disease35.
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical tests were conducted two-sided and a p-value < 0.05 was considered to indicate statistical significance
Data availability
YRHCD is open for research purposes. Data requests should be sent to the Yinzhou District Center for Disease Control and Prevention, by contacting Mr. Peng Shen at shen-peng@foxmail.com or Mr. Yexiang Sun at 19464337@qq.com. The feasibility, novelty, and scientific rigor of the proposal will be discussed, and the decision whether to share the data will be made by an academic committee. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code for this study is available in a ResearchGate repository (https://www.researchgate.net/publication/363771107_Code_TZD_PD).
References
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Ascherio, A. P. & Schwarzschild, M. A. P. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272 (2016).
Cardoso, S. & Moreira, P. I. Antidiabetic drugs for Alzheimer’s and Parkinson’s diseases: Repurposing insulin, metformin, and thiazolidinediones. Int Rev. Neurobiol. 155, 37–64 (2020).
Cheong, J., de Pablo-Fernandez, E., Foltynie, T. & Noyce, A. J. The Association between type 2 diabetes mellitus and Parkinson’s disease. J. Parkinsons Dis. 10, 775–789 (2020).
International Diabetes Federation. IDF Diabetes Atlas 9th edition 2019. Brussels, Belgium 2019. https://www.diabetesatlas.org/upload/resources/2019/IDF_Atlas_9th_Edition_2019.pdf.
Brauer, R. et al. Diabetes medications and risk of Parkinson’s disease: a cohort study of patients with diabetes. Brain 143, 3067–3076 (2020).
Aviles-Olmos, I., Limousin, P., Lees, A. & Foltynie, T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain 136, 374–384 (2013).
Meléndez-Flores, J. D., Millán-Alanís, J. M., González-Martínez, A., Álvarez-Villalobos, N. A. & Estrada-Bellmann, I. Does glitazone treatment have a role on the prevention of Parkinson’s disease in adult diabetic population? A systematic review. Metab. Brain Dis. 35, 1067–1075 (2020).
Hussain, S. et al. Thiazolidinedione use is associated with reduced risk of Parkinson’s disease in patients with diabetes: a meta-analysis of real-world evidence. Neurol. Sci. 41, 3697–3703 (2020).
Lim, S. Y. et al. Parkinson’s disease in the Western Pacific Region. Lancet Neurol. 18, 865–879 (2019).
Qi, S. et al. Prevalence of Parkinson’s disease: a community-based study in China. Mov. Disord. 36, 2940–2944 (2021).
GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Wang, Y. et al. Neuroprotective effect and mechanism of thiazolidinedione on dopaminergic neurons in vivo and in vitro in Parkinson’s disease. Ppar Res. 2017, 4089214 (2017).
Xing, B., Xin, T., Hunter, R. L. & Bing, G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J. Neuroinflammation. 5, 4 (2008).
Carta, A. R. et al. Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 194, 250–261 (2011).
Breidert, T. et al. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J. Neurochem. 82, 615–624 (2002).
Brauer, R. et al. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med. 12, e1001854 (2015).
Brakedal, B. et al. Glitazone use associated with reduced risk of Parkinson’s disease. Mov. Disord. 32, 1594–1599 (2017).
Chang, Y. H., Yen, S. J., Chang, Y. H., Wu, W. J. & Lin, K. D. Pioglitazone and statins lower incidence of Parkinson disease in patients with diabetes mellitus. Eur. J. Neurol. 28, 430–437 (2021).
Lin, H. L., Lin, H. C., Tseng, Y. F., Chao, J. C. & Hsu, C. Y. Association of thiazolidinedione with a lower risk of Parkinson’s disease in a population with newly-diagnosed diabetes mellitus. Ann. Med. 50, 430–436 (2018).
Wu, H. F. et al. Pioglitazone use and Parkinson’s disease: a retrospective cohort study in Taiwan. BMJ Open. 8, e23302 (2018).
Sunnarborg, K. et al. Association between different diabetes medication classes and risk of Parkinson’s disease in people with diabetes. Pharmacoepidemiol Drug Saf. 31, 875–882 (2022).
Rhee, S. Y. et al. Association between glycemic status and the risk of Parkinson disease: a nationwide population-based study. Diabetes Care. 43, 2169–2175 (2020).
Connolly, J. G., Bykov, K. & Gagne, J. J. Thiazolidinediones and Parkinson disease: a cohort study. Am. J. Epidemiol. 182, 936–944 (2015).
Lund, J. L., Richardson, D. B. & Stürmer, T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr. Epidemiol. Rep. 2, 221–228 (2015).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 35, 2665–2673 (2012).
Zhao, H. et al. Sulfonylurea and cancer risk among patients with type 2 diabetes: a population-based cohort study. Front. Endocrinol. 13, 874344 (2022).
Yoshida, K., Solomon, D. H. & Kim, S. C. Active-comparator design and new-user design in observational studies. Nat. Rev. Rheumatol. 11, 437–441 (2015).
NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 14, 795–803 (2015).
Lin, H. et al. Using big data to improve cardiovascular care and outcomes in China: a protocol for the CHinese Electronic health Records Research in Yinzhou (CHERRY) Study. BMJ Open. 8, e19698 (2018).
Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin. J. Diabetes Mellit. 10, 4–67 (2018).
Bannay, A. et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care. 54, 188–194 (2016).
Austin, P. C. & Stuart, E. A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34, 3661–3679 (2015).
von Hippel, P. T. How many imputations do you need? A two-stage calculation using a quadratic rule. Socio. Method Res. 49, 699–718 (2020).
Danaei, G., Rodríguez, L. A. G., Cantero, O. F., Logan, R. & Hernán, M. A. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat. Methods Med Res. 22, 70–96 (2013).
Acknowledgements
All authors gratefully acknowledge funding support from the China Postdoctoral Science Foundation (grant NO. 2022M710251) and the National Natural Science Foundation of China (Grant No. 81973146).
Author information
Authors and Affiliations
Contributions
H.Z. conceived of and designed the work. H.L., P.S., and Y.S. acquired the data. H.Z. analyzed the data. H.Z. drafted the manuscript. L.Z. and S.Z. critically revised the manuscript for important intellectual content. S.Z., H.L., and P.S. supervised the study. H.Z. and S.Z. obtained the funding. All authors were responsible for the interpretation of the data, and revised, and gave final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, H., Zhuo, L., Sun, Y. et al. Thiazolidinedione use and risk of Parkinson’s disease in patients with type 2 diabetes mellitus. npj Parkinsons Dis. 8, 138 (2022). https://doi.org/10.1038/s41531-022-00406-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00406-8
This article is cited by
-
Mitochondrial CISD1/Cisd accumulation blocks mitophagy and genetic or pharmacological inhibition rescues neurodegenerative phenotypes in Pink1/parkin models
Molecular Neurodegeneration (2024)
-
A crazy trio in Parkinson's disease: metabolism alteration, α-synuclein aggregation, and oxidative stress
Molecular and Cellular Biochemistry (2024)
-
Do oral antidiabetic medications alter the risk of Parkinson’s disease? An updated systematic review and meta-analysis
Neurological Sciences (2023)