Abstract
Autonomic dysregulation in Parkinson’s disease (PD) can precede motor deficits and is associated with reduced quality of life, disease progression, and increased mortality. Objective markers of autonomic involvement in PD are limited. Corneal confocal microscopy (CCM) is a rapid ophthalmic technique that can quantify small nerve damage in a range of peripheral and autonomic neuropathies. Here we investigated whether CCM can be used to assess autonomic symptoms in PD. Based on the scale for outcomes in Parkinson’s disease for autonomic symptoms (SCOPA-AUT), patients with PD were classified into those without autonomic symptoms (AutD-N), with single (AutD-S), and multiple (AutD-M) domain autonomic dysfunction. Corneal nerve fiber pathology was quantified using CCM, and the relationship with autonomic symptoms was explored. The study enrolled 71 PD patients and 30 control subjects. Corneal nerve fiber density (CNFD), corneal nerve branch density (CNBD), corneal nerve fiber length (CNFL), and CNBD/CNFD ratio were lower in PD patients with autonomic symptoms compared to those without autonomic symptoms. Autonomic symptoms correlated positively with CNFD (r = −0.350, p = 0.004), and were not related to Levodopa equivalent daily dose (r = 0.042, p = 0.733) after adjusting for age, disease severity, disease duration or cognitive function. CCM parameters had high sensitivity and specificity in distinguishing patients with PD with and without autonomic symptoms. PD patients with autonomic symptoms have corneal nerve loss, and CCM could serve as an objective ophthalmic imaging technique to identify patients with PD and autonomic symptoms.
Similar content being viewed by others

Introduction
Parkinson’s disease (PD) is a complex neurological disorder that can present with motor and non-motor symptoms1, although motor symptoms such as tremors and bradykinesia are the main reason for patients to seek medical advice2. Rapid eye movement sleep behavior disorder, olfactory deficits, and signs and symptoms of autonomic dysregulation can precede motor deficits and could therefore be targeted as prodromal or diagnostic biomarkers in PD3,4.
Although PD is traditionally regarded as a central neurodegenerative disease (CNS), peripheral nerve involvement is increasingly recognized5,6. Large fiber (Aα/β fibers) involvement may be related to levodopa administration, but small fiber (Aδ and C fibers) neuropathy is thought to be intrinsic to the neurodegenerative process in PD7. Moreover, epidemiological and experimental studies suggest that the dysfunctional autonomic innervation in the gut, heart, and skin may provide a route by which Parkinson’s disease pathology spreads both to and from the CNS8. Large fiber neuropathy is usually diagnosed with nerve conduction studies, and small fiber neuropathy can be assessed in skin biopsy9. Reliable and easy-to-use tests of autonomic integrity may be critical in early diagnosis and to assess the impact of interventions that prevent neurodegeneration in PD.
Multiple studies have shown that corneal confocal microscopy (CCM) can be used to quantify corneal nerve loss, has good diagnostic utility for diabetic neuropathy10, and predicts the development of diabetic neuropathy11. Furthermore, corneal nerve loss has very high sensitivity and specificity and has been related to the severity of diabetic autonomic neuropathy12. An increasing number of studies have shown evidence of corneal nerve loss in PD patients13, which has been associated with motor and non-motor symptoms14,15. Furthermore, in a longitudinal study, Lim et al showed that greater corneal nerve loss was associated with more rapid motor progression in a cohort of patients with PD14. We have also recently shown that the severity of corneal nerve loss was associated with the severity of cognitive dysfunction in PD15.
In the present study, the relationship between corneal nerve loss, quantified using CCM, and the severity of autonomic symptoms was evaluated with the scale for outcomes in Parkinson’s disease for autonomic symptoms (SCOPA-AUT). The diagnostic utility of CCM for autonomic symptoms in PD was also established.
Results
Clinical profiles
Of 84 PD patients enrolled, 71 underwent complete investigations. Eight patients with impaired glucose tolerance, 3 with corneal disease, and 2 with a suspected diagnosis of multiple system atrophy were excluded. The age of the PD group (44.62% male) was 62.59 ± 7.75 years old, with an age at onset 58.69 ± 8.12 years and a disease duration of 3 (2, 4) years. The average H-Y stage of the PD group was 2 (1,3) and was comprised of H-Y I (21, 29.58%), H-Y II (21, 29.58%), H-Y III (14, 19.72%), and H-Y IV (15, 21.13%), respectively. In the control group (n = 30), 53.33% were male, with an average age of 62.43 ± 6.16 years.
Autonomic symptoms
Of the 71 PD patients, 14 (19.7%) had no autonomic symptoms (AutD-N), 14 (19.7%) had autonomic symptoms in one domain (AutD-S), and 43 (60.6%) had autonomic symptoms in two or/more domains (AutD-M). The demographic and clinical profiles of each subgroup are presented in Table 1. The SCOPA-AUT score in each group was 0 (AutD-N), 6.93 ± 2.87 (AutD-S), and 15.93 ± 6.47 (AutD-M), respectively. The relative frequency of domains affected: urinary (52), gastrointestinal (43), thermoregulatory (32), cardiovascular (20), pupillomotor (17), and sexual (5) is depicted in Fig. 1a. Urinary complaints were primarily incontinence, incomplete emptying and increased frequency, whilst gastrointestinal complaints included constipation, swallowing difficulties and sialorrhea. Other autonomic symptoms include hyperhidrosis (thermoregulatory), light-headiness when standing up (cardiovascular), and oversensitivity to bright light (pupillomotor). Erectile dysfunction and retrograde ejaculation in men and reduced vaginal lubrication and orgasm were reported in females. Orthostatic hypotension was reported in 22 patients (30.98%): 4 (28.57%) in AutD-S group and 18 (41.86%) in the AutD-M group. In the AutD-M group, the numbers of patients with involvement of 2, 3, 4, 5, and 6 domains were 8, 12, 14, 6, and 3, respectively (Fig. 1b).
Corneal nerve fiber parameters
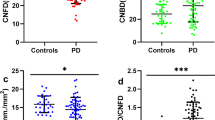
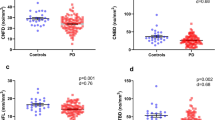
There was a progressive loss of CNFD, CNBD, and CNFL with increasing severity of autonomic dysfunction (Figs. 2 and 3). CNFD in AutD-N (no./mm2, 30.88 ± 2.42 vs 34.33 ± 3.78; mean difference, 3.45; 95% CI, 0.29–6.59; P = 0.024, by one-way ANOVA), AutD-S (no./mm2, 27.87 ± 3.11 vs 34.33 ± 3.78; mean difference, 6.46; 95% CI, 3.31–9.61; P < 0.001, by one-way ANOVA) and AutD-M (no./mm2, 23.63 ± 3.93 vs 34.33 ± 3.78; mean difference, 10.70; 95% CI, 8.39–13.01; P < 0.001, by one-way ANOVA) groups were significantly lower compared to controls. CNFD in AutD-M was significantly lower than in patients with AutD-S (no./mm2, 23.63 ± 3.93 vs 27.87 ± 3.11; mean difference, 4.24; 95% CI, 1.25–7.23; P = 0.001) and AutD-N (no./mm2, 23.63 ± 3.93 vs 30.88 ± 2.42; mean difference, 7.25; 95% CI, 4.26–10.25; P < 0.001).
Corneal nerve fibers are beaded, linear homogeneous and highly reflective (a–d). Nerve fiber trunks are highlighted in red, green dots indicate the origin of the branches, and blue and red lines combined indicate the corneal nerve fiber length (e–h). Pictures (e–h) were analyzed with the manual software (CCMetrics) and images (i–l) were marked with the automated version (ACCMetrics). CCM corneal confocal microscopy, PD Parkinson’s disease, AutD-N patients with no autonomic symptoms, AutD-S patients with single-domain autonomic symptoms, AutD-M patients with multiple-domain autonomic symptoms. Scale bar = 100 um.
CNFD, CNBD, CNFL, and CNBD/CNFD ratio in AutD-N, AutD-S, and AutD-M groups were compared with controls (a–d). Errors bars represent mean ± standard deviation (*P < 0.05, **P < 0.01, ***P < 0.001). PD Parkinson’s disease, AutD-N patients with no autonomic symptoms, AutD-S patients with single-domain autonomic symptoms, AutD-M patients with multiple-domain autonomic symptoms, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length.
CNBD in AutD-N (no./mm2, 40.22(34.92, 58.05) vs 24.58 ± 8.23; P < 0.001, by Kruskal–Wallis test) and AutD-S (no./mm2, 37.52 ± 7.61 vs 24.58 ± 8.23; P = 0.003, by Kruskal–Wallis test) was significantly higher compared to controls. AutD-S [no./mm2, 37.52 ± 7.61 vs 40.22 (34.95, 58.05); P = 0.007] and AutD-M [no./mm2, 25.92 ± 10.60 vs 40.22 (34.95, 58.05); P < 0.001] had a lower CNBD compared to the AutD-N group.
CNFL was significantly lower in the AutD-M compared to the AutD-S (mm/mm2, 12.85 ± 2.55 vs 15.74 ± 1.56; mean difference, 2.89; 95% CI, 0.99–4.79; P < 0.001), AutD-N (mm/mm2, 12.85 ± 2.55 vs 17.54 ± 2.03; mean difference, 4.69; 95% CI, 2.79–6.59; P < 0.001), and control (mm/mm2, 12.85 ± 2.55 vs 15.86 ± 2.27; mean difference, 3.02; 95% CI, 1.55–4.48; P < 0.001) group.
The CNBD/CNFD ratio was higher in all three PD groups compared to controls, with no significant difference between the subgroups with PD.
Correlations between autonomic symptoms and clinical and CCM parameters
SCOPA-AUT was negatively associated with CNFD (r = −0.350, P = 0.004) after adjusting for confounders including age, disease severity, disease duration, and MoCA scores. There was no correlation between SCOPA-AUT and LEDD (r = 0.042, p = 0.733) (Fig. 4). CNFD (23.81 ± 2.95 vs 26.55 ± 4.83; t = 2.202, P = 0.031) was significantly lower, while CNBD (28.65 ± 10.94 vs 33.01 ± 13.57; t = 1.206, P = 0.232) and CNFL (13.74 ± 2.19 vs 14.53 ± 3.19; t = 0.960, P = 0.340) were comparable between PD patients with and without pupillary oversensitivity. Furthermore, patients with pupillary oversensitivity were all from the AutD-S and AutD-M groups and had a higher SCOPA-AUT score (17.59 ± 6.68 vs 8.94 ± 7.72; t = 4.151, P < 0.001) than patients without oversensitivity. After adjustment for SCOPA-AUT there was no significant difference in CNFD (P = 0.878), CNBD (P = 0.801), or CNFL (P = 0.237) between the two groups.
ROC analysis
ROC analysis showed that CNFD, CNBD, and CNFL could distinguish between AutD-S and AutD-N with an area under the curve (AUC) of 81.89% (95% CI, 64.75–99.02%), 68.37% (95% CI, 48.45–88.28%), and 76.53% (95% CI, 58.72–94.34%), respectively. Using a CNFD cutoff of <29.84 no./mm2, the sensitivity and specificity for AutD-S was 85.71% and 78.57%. Using a CNBD cutoff of <37.94 no./mm2, the sensitivity and specificity for AutD-S was 71.48% and 64.29%. Using a CNFL cutoff of <17.06 mm/mm2, the sensitivity and specificity for AutD-S was 85.71% and 64.29%. A combination of all three corneal nerve parameters increased the AUC to 87.24% (95% CI, 58.72–94.34%), with a sensitivity and specificity of 85.71% and 92.86% (Fig. 5a).
a, b PD Parkinson’s disease, AutD-N patients with no autonomic symptoms, AutD-S patients with single-domain autonomic symptoms, AutD-M patients with multiple-domain autonomic symptoms, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length, AUC area under the curve.
The AUC distinguishing AutD-M from AutD-S for CNFD, CNBD, and CNFL was 83.72% (95% CI, 72.34–95.10%), 79.07% (95% CI, 67.42–90.72%), 83.31% (95% CI, 72.36–94.25%), respectively. Using a CNFD cutoff of <25.83 no./mm2, the sensitivity and specificity for AutD-M were 69.77% and 85.71%. Using a CNBD cutoff of <29.18 no./mm2, the sensitivity and specificity for AutD-M were 65.12% and 92.86%. Using a CNFL cutoff of <13.91 mm/mm2, the sensitivity and specificity for AutD-M were 65.12% and 92.86%. A combination of all three corneal nerve parameters increased the AUC to 91.53% (95% CI, 83.99–99.06%) with a sensitivity and specificity of 79.07% and 92.86%, respectively (Fig. 5b).
Discussion
In the present study, we show that corneal nerve loss is associated with the severity of autonomic symptoms in patients with PD. Autonomic symptoms are an important and under-recognized area of functional disability that can severely affect the quality of life in patients with Parkinson’s disease. Delayed gastric emptying can lead to impaired drug absorption with the “delayed ON” or even “no ON” phenomenon interfering with the therapeutic effect of dopaminergic medication, worsening motor function. Orthostatic hypotension can cause syncope and falls16 and fall-related fractures, pneumonia, and even death. Some autonomic symptoms, such as constipation, can occur in the early stage of disease and may even precede the onset of motor symptoms by many years16,17. We speculate that both dopaminergic and adrenergic neurons in the nigrostriatal and peripheral nervous systems are lost progressively with the gradual worsening of motor and non-motor symptoms. Moreover, autonomic nerve dysfunction has been associated with faster disease progression and shorter survival18, thus timely and accurate detection of autonomic deficits is important for PD prognosis and management.
Autonomic nerve fibers are thinly myelinated or unmyelinated nerve fibers, but damage to these fibers is difficult to quantify. Pathological examination of skin biopsies has shown decreased intraepidermal nerve fiber density (IENFD)19 and a relationship between mean axonal length and total nerve fiber length with motor and autonomic symptoms and autonomic dysfunction20. Additionally, there is evidence of a non-length dependent distribution of phosphorylated α-synuclein in autonomic fibers in the skin of patients with PD21,22 with differences between patients with PD and multiple system atrophy23. While skin biopsy provides important insights in the study of autonomic neuropathies, it is invasive and requires complex immunostaining protocols in specialized laboratories24,25. Quantitative sensory testing (QST) is non-invasive and easily performed, but is subjective and can be highly variable. Indeed, a recent deep phenotyping study using the standardized German Research Network on Neuropathic pain protocol showed no differences between controls and drug-naïve PD patients26.
Cardiac 123I-MIBG scintigraphy27 and intestinal 11C-donepezil PET/CT28 can identify sympathetic denervation and impairment of parasympathetic terminals in PD patients, but these techniques are expensive and not readily available.
In this large cohort of patients with PD we show evidence of a proximal loss of corneal nerves as evidenced by a progressive reduction in CNFD, which was associated with the severity of autonomic symptoms. This is consistent with the findings in our small pilot study in 26 PD patients, where we also showed a correlation between corneal nerve loss and autonomic symptoms (SCOPA-AUT) and function (Deep breathing-Heart rate variability)13. Indeed, in the present study, we show that PD patients with pupillary oversensitivity have a lower CNFD, which is no longer significant after adjustment for the SCOPA-AUT score, further supporting a relationship between CCM and autonomic abnormalities. Corneal nerve loss has been associated with autonomic neuropathy in amyloid neuropathy29, fibromyalgia30, and diabetic neuropathy12,31. The increase in corneal nerve branches and length has been found in several previous studies in patients with PD13,15 and may represent nerve regeneration, especially in the earlier phases of the disease, as evidenced by the higher CNBD and CNFL in the AutD-N and AutD-S groups compared to the AutD-M group. To further assess the interplay between proximal nerve degeneration and distal nerve regeneration, we quantified the CNBD/CNFD ratio. While there was a trend for a decrease in the ratio with increasing severity of autonomic symptoms, this was not significant, suggesting a dynamic and complex process that requires careful interpretation in future studies.
We found no correlation between SCOPA-AUT and LEDD, suggesting that autonomic involvement in PD reflects intrinsic neurodegeneration and is consistent with our recent study, where we also showed no association between CNFD and LEDD15.
Corneal nerve loss assessed using CCM is evident even in patients without autonomic symptoms indicating sub-clinical deficits detected using CCM, which then progress with increasing severity of autonomic neuropathy. Indeed, CCM detected corneal nerve loss when IENFD was still normal in drug-naïve patients with Parkinson’s disease32. Previous studies suggest that the SCOPA-AUT scores correlated with age33,34, disease duration34, disease severity35, and cognitive function36. Therefore, we adjusted these confounders in the relation analysis between autonomic function and CNFD. Our study shows SCOPA-AUT related positively to CNFD.
The relatively good diagnostic outcomes to differentiate patients with minimal and more prominent autonomic symptoms from those without autonomic symptoms using individual and especially combined corneal nerve parameters argues in favor of the diagnostic utility of CCM in patients with PD and autonomic deficits. This adds to the diagnostic utility of CCM in PD as we have also recently shown that it has a good ability to differentiate PD patients with and without cognitive dysfunction15. Although this is a good-sized cohort of patients with PD, the cross-sectional design cannot infer causality. Longitudinal studies are required to assess if CCM can identify patients with a faster deterioration of autonomic symptoms and poorer prognosis as has been shown for motor progression recently14. We acknowledge that SCOPA-AUT is a subjective symptom questionnaire36,37,38, although studies have shown that it reflects the severity of autonomic dysfunction in PD39,40. We also acknowledge that the presence of cognitive dysfunction may limit the accuracy of the assessment of SCOPA-AUT.
In conclusion, this study shows an association between corneal nerve loss assessed using CCM with the presence of autonomic symptoms and an excellent diagnostic utility for identifying PD patients with autonomic symptoms. Therefore, CCM represents a safe, rapid, and convenient in vivo ophthalmic imaging technique to identify patients with PD and autonomic symptoms. These findings warrant longitudinal studies to define the prognostic utility of CCM in PD.
Materials and methods
Subjects
The study was approved by the ethics committee of Henan Provincial People’s Hospital. Patients with PD were recruited from Henan Provincial People’s Hospital between March 2017 and January 2020. All subjects agreed to participate in the study, and written informed consent was obtained.
Age and sex were assessed in all subjects. PD was diagnosed according to the 2015 Movement Disorder Society clinical diagnostic criteria for Parkinson’s disease41. Clinically established PD and clinically probable PD were included. Atypical parkinsonism such as progressive supranuclear palsy, cortical basal ganglia degeneration, multiple system atrophy, and secondary parkinsonism (drug-induced, immune-mediated, inflammatory, vascular, infectious, traumatic or neoplasm, etc.) was excluded from the study. Healthy controls were included from either volunteers or spouses of PD patients who had no history of movement disorder or cognitive impairment. For the investigational purpose, patients or healthy controls younger than 40 or older than 85 years of age were excluded from the study. Participants with a history of eye surgery, eye inflammation, glaucoma, corneal disease, or thyroid eye disease were excluded. Other causes of peripheral neuropathy were excluded by a history of excess alcohol use (>150 ml/day) and an assessment of vitamin B12 and folate, serum electrophoresis to exclude multiple myeloma, cryoglobulinemia, macroglobulinemia, and oral glucose tolerance test to exclude impaired glucose tolerance and diabetes. To increase diagnostic accuracy, the clinical profiles of each participant were carefully reviewed by two experienced neurologists (J.-J. Ma and H.-Q. Yang) who specialized in movement disorders.
Clinical evaluation
The evaluation of motor and non-motor symptoms was all performed in the “ON” state in PD patients. Motor function was assessed with part I, II, III, and IV sub-scales of the unified Parkinson’s disease rating scale (UPDRS), and Hoehn and Yahr (H-Y) staging was undertaken for all patients42. Disease duration was defined as the time between presentation with first motor symptoms and enrollment into the present study. Montreal cognitive assessment (MoCA, Beijing Version) was used to assess the cognitive status. Anxiety and depressive symptoms were evaluated with the 14-item Hamilton anxiety (HAMA-14) rating scale and the 24-item Hamilton depression (HAMD-24) rating scale, respectively. Levodopa equivalent daily dose (LEDD) was assessed according to the levodopa conversion formula43. Briefly, 100 mg levodopa = 133 mg entacapone = 1 mg pramipexole = 5 mg ropinirole = 10 mg selegiline = 1 mg rasagiline = 100 mg amantadine.
Autonomic symptom severity
The SCOPA-AUT, a reliable and validated questionnaire that evaluates autonomic symptoms, was undertaken in patients with PD44. Briefly, 27-items with six domain rating scales were used to evaluate autonomic symptoms (item 1–7 for the gastrointestinal tract, item 8–13 for urinary tract, item 14–16 for the cardiovascular system, item 17,18,20,21 for thermoregulation, item 19 for pupil activity, item 22–24 for male sexual function, item 25–26 for female sexual dysfunction; and item 27, treatment of either of above-mentioned symptoms). Each item is given a score, with a higher score indicating more severe autonomic dysfunction. PD patients were divided into three subgroups according to domain autonomic symptom37. PD patients with no autonomic symptoms were defined as AutD-N; PD patients with autonomic symptoms in one domain were defined as AutD-S; PD patients with autonomic symptoms in two or/more domains were defined as AutD-M. Orthostatic hypotension was defined as a drop of systolic blood pressure (≥20 mm Hg) or diastolic blood pressure (≥10 mm Hg) within 3 minutes of standing from a supine position45.
Corneal confocal microscopy
A Heidelberg Retina Tomograph III with a Rostock Cornea Module (HRT III RCM; Heidelberg Engineering GmbH, Heidelberg, Germany) was used to acquire images of the central corneal sub-basal nerve plexus. Topical lidocaine was used to anesthetize the eye of each subject, and they were seated comfortably and instructed to fixate on an outer fixation light. The TomoCap was correctly positioned on the apex of the cornea by visualizing it with the CCD camera. An experienced examiner took images at the level of the sub-basal nerve plexus in the central cornea using the “section” mode according to an established protocol46. Four to six best-quality CCM images from the central cornea of each eye were selected and analyzed using a validated, manual (CCMetrics) and automated (ACCMetrics, Imaging Science and Biomedical Engineering, Manchester, UK) purpose-written software47. Three parameters were analyzed: (a) corneal nerve fiber density (CNFD): the number of main nerve fibers per square millimeter; (b) corneal nerve fiber branch density (CNBD): the number of primary branches originating from the main nerve; and (c) corneal nerve fiber length (CNFL): the sum of the length of all nerve fibers per square millimeter. The CNBD/CNFD ratio was calculated to assess nerve regenerative capacity15.
Data analysis
The normality of data was assessed by the Shapiro-Wilk test. For normally distributed variables, numbers are expressed as mean ± standard deviation (SD). Analysis of variance with Bonferroni as post hoc test was used for multiple group comparison. For non-normal or non-homoscedasticity variables, numbers are expressed as median (interquartile range). The nonparametric Kruskal–Wallis test was used for multiple comparisons. Chi-square tests and Fisher’s exact tests were used to compare categorical variables. Partial correlation analysis was performed in PD to assess the association between SCOPA-AUT scores and corneal nerve parameters and clinical characteristics, adjusting for confounders. The ROC curve was used to analyze the capability of CNFD, CNBD, and CNFL for distinguishing PD patients with single-domain autonomic impairment from no autonomic impairment, and multiple-domain autonomic impairment from single-domain autonomic impairment. All analyses were carried out using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Dot plots and ROC curves were generated using GraphPad Prism version 8.0 (GraphPad Software, Inc, San Diego, CA, USA). P < 0.05 was considered statistically significant.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Camacho, M. et al. Early constipation predicts faster dementia onset in Parkinson’s disease. npj Parkinsons Dis. 7, 45 (2021).
Pol, F., Salehinejad, M. A., Baharlouei, H. & Nitsche, M. A. The effects of transcranial direct current stimulation on gait in patients with Parkinson’s disease: a systematic review. Transl. Neurodegener. 10, 22 (2021).
He, S. et al. Several miRNAs derived from serum extracellular vesicles are potential biomarkers for early diagnosis and progression of Parkinson’s disease. Transl. Neurodegener. 10, 25 (2021).
Postuma, R. B. & Berg, D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 12, 622–634 (2016).
Comi, C. et al. Peripheral nervous system involvement in Parkinson’s disease: evidence and controversies. Parkinsonism Relat. Disord. 20, 1329–1334 (2014).
Che, N. N. & Yang, H. Q. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl. Neurodegener. 9, 28 (2020).
Ceravolo, R. et al. Neuropathy and levodopa in Parkinson’s disease: evidence from a multicenter study. Mov. Disord. 28, 1391–1397 (2013).
Sharabi, Y., Vatine, G. D. & Ashkenazi, A. Parkinson’s disease outside the brain: targeting the autonomic nervous system. Lancet Neurol. 20, 868–876 (2021).
Cossu, G. & Melis, M. The peripheral nerve involvement in Parkinson disease: a multifaceted phenomenon. Parkinsonism Relat. Disord. 25, 17–20 (2016).
Gad, H. et al. Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: a systematic review and meta-analysis. J. Diabetes Investig. 13, 134–147 (2021).
Perkins, B. A. et al. Corneal confocal microscopy predicts the development of diabetic neuropathy: a longitudinal diagnostic multinational consortium study. Diabetes Care 44, 2107–2114 (2021).
Tavakoli, M., Begum, P., McLaughlin, J. & Malik, R. A. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve 52, 363–370 (2015).
Kass-Iliyya, L. et al. Small fiber neuropathy in Parkinson’s disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat. Disord. 21, 1454–1460 (2015).
Lim, S. H. et al. Corneal confocal microscopy identifies parkinson’s disease with more rapid motor progression. Mov. Disord. 36, 1927–1934 (2021).
Che, N. N. et al. Corneal nerve fiber loss relates to cognitive impairment in patients with Parkinson’s disease. npj Parkinsons Dis. 7, 80 (2021).
Dommershuijsen, L. J. et al. Orthostatic hypotension: a prodromal marker of Parkinson’s disease? Mov. Disord. 36, 164–170 (2021).
Yoo, S. W. et al. Delayed orthostatic hypotension in Parkinson’s disease. npj Parkinsons Dis. 7, 37 (2021).
De Pablo-Fernandez, E. et al. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol. 74, 970–976 (2017).
Donadio, V. et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 82, 1362–1369 (2014).
Jeziorska, M. et al. Increased intraepidermal nerve fiber degeneration and impaired regeneration relate to symptoms and deficits in Parkinson’s disease. Front. Neurol. 10, 111 (2019).
Melli, G. et al. Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann. Clin. Transl. Neurol. 5, 1394–1407 (2018).
Donadio, V. et al. Skin nerve misfolded alpha-synuclein in pure autonomic failure and Parkinson disease. Ann. Neurol. 79, 306–316 (2016).
Haga, R. et al. Clinical utility of skin biopsy in differentiating between Parkinson’s disease and multiple system atrophy. Parkinsons Dis. 2015, 167038 (2015).
Christopher, H. et al. Skin biopsy in evaluation of autonomic disorders. Continuum (Minneap. Minn.) 26, 200–212 (2020).
Wang, N., Garcia, J., Freeman, R. & Gibbons, C. H. Phosphorylated alpha-synuclein within cutaneous autonomic nerves of patients with Parkinson’s disease: the implications of sample thickness on results. J. Histochem. Cytochem. 68, 669–678 (2020).
Frundt, O. et al. Quantitative sensory testing (QST) in drug-naive patients with Parkinson’s disease. J. Parkinsons Dis. 9, 369–378 (2019).
Giannoccaro, M. P. et al. Comparison of 123I-MIBG scintigraphy and phosphorylated alpha synuclein skin deposits in synucleinopathies. Parkinsonism Relat. Disord. 81, 48–53 (2020).
Knudsen, K. et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 17, 618–628 (2018).
Rousseau, A. et al. Potential role of in vivo confocal microscopy for imaging corneal nerves in transthyretin familial amyloid polyneuropathy. JAMA Ophthalmol. 134, 983–989 (2016).
Ramirez, M. et al. Correlation between corneal nerve density and symptoms of small fiber neuropathy in patients with fibromyalgia: the confounding role of severe anxiety or depression. J. Clin. Rheumatol. 27, e606–e608 (2020).
Misra, S. L. et al. In vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci. 56, 5060–5065 (2015).
Podgorny, P. J., Suchowersky, O., Romanchuk, K. G. & Feasby, T. E. Evidence for small fiber neuropathy in early Parkinson’s disease. Parkinsonism Relat. Disord. 28, 94–99 (2016).
Verbaan, D. et al. Patient-reported autonomic symptoms in Parkinson disease. Neurology 69, 333–341 (2007).
Rodriguez-Blazquez, C. et al. Independent validation of the scales for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT). Eur. J. Neurol. 17, 194–201 (2010).
Kim, J. Y. et al. Validation of the Korean version of the scale for outcomes in Parkinson’s disease-autonomic. J. Mov. Disord. 10, 29–34 (2017).
Dayan, E., Sklerov, M. & Browner, N. Disrupted hypothalamic functional connectivity in patients with PD and autonomic dysfunction. Neurology 90, e2051–e2058 (2018).
Merola, A. et al. Autonomic dysfunction in Parkinson’s disease: a prospective cohort study. Mov. Disord. 33, 391–397 (2018).
Xu, X. et al. Clinical utility of SUDOSCAN in predicting autonomic neuropathy in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 64, 60–65 (2019).
Chen, H. et al. The compensatory phenomenon of the functional connectome related to pathological biomarkers in individuals with subjective cognitive decline. Transl. Neurodegener. 9, 21 (2020).
Evatt, M. L. et al. Dysautonomia rating scales in Parkinson’s disease: sialorrhea, dysphagia, and constipation–critique and recommendations by movement disorders task force on rating scales for Parkinson’s disease. Mov. Disord. 24, 635–646 (2009).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Goetz, C. G. et al. Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Visser, M., Marinus, J. & Stiggelbout, A. M. & Van Hilten, J. J. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov. Disord. 19, 1306–1312 (2004).
Lahrmann, H. et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur. J. Neurol. 13, 930–936 (2006).
Tavakoli, M. & Malik, R. A. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J. Vis. Exp. 3, 2194 (2011).
Tavakoli, M. et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care 38, 838–843 (2015).
Acknowledgements
We thank the patients and healthy individuals for their participation in this study. We also thank the Manchester Biomedical Research Centre for access to the CCMetrics and ACCMetrics software. The study was funded by Talent project of Henan Provincial People’s Hospital (23456-4), Henan Province Science and Technology Development Plan (192102310085), and Henan Province Medical Science and Technology Research Program (SBGJ202102035).
Author information
Authors and Affiliations
Contributions
N.-N.C., S.C., and Q.-H.J. made contributions to conception and design, acquisition of data, and drafting the manuscript. S.-Y.C. and Z.-X.Z. participated in data collection. X.L. and J.-J.M. contributed in interpretation of the data. R.A.M. helped critically in data analysis and revised the manuscript. H.-Q.Y. undertook supervision of the research group, acquisition of funding, and was involved in critically revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Che, NN., Chen, S., Jiang, QH. et al. Corneal confocal microscopy differentiates patients with Parkinson’s disease with and without autonomic involvement. npj Parkinsons Dis. 8, 114 (2022). https://doi.org/10.1038/s41531-022-00387-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00387-8
This article is cited by
-
Evaluation of peripheral and autonomic nervous systems dysfunctions in patients with Parkinson’s disease
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
Corneal confocal microscopy may help to distinguish Multiple System Atrophy from Parkinson’s disease
npj Parkinson's Disease (2024)