Abstract
Previous studies have shown less access to deep brain stimulation (DBS) for Parkinson’s disease (PD) in women compared to men raising concerns about a potential gender gap resulting from nonclinical factors or gender differences in clinical efficacy for postoperative quality of life (QoL), motor, and nonmotor symptoms (NMS) outcomes. This was a cross-sectional and a longitudinal, prospective, observational, controlled, quasi-experimental, international multicenter study. A total sample size of 505 consisted of 316 consecutively referred patients for DBS indication evaluation at the University Hospital Cologne (01/2015–09/2020) and 189 consecutively treated patients at DBS centers in the University Hospitals Cologne and Marburg, Salford’s Royal Hospital Manchester, and King’s College Hospital London. In the cross-sectional cohort, we examined gender proportions at referral, indication evaluations, and DBS surgery. In the longitudinal cohort, clinical assessments at preoperative baseline and 6-month follow-up after surgery included the PD Questionnaire-8, NMSScale, Scales for Outcomes in PD-motor scale, and levodopa-equivalent daily dose. Propensity score matching resulted in a pseudo-randomized sub-cohort balancing baseline demographic and clinical characteristics between women with PD and male controls. 316 patients were referred for DBS. 219 indication evaluations were positive (women n = 102, respectively n = 82). Women with PD were disproportionally underrepresented in referrals compared to the general PD population (relative risk [RR], 0.72; 95%CI, 0.56–0.91; P = 0.002), but more likely to be approved for DBS than men (RR, 1.17; 95%CI, 1.03–1.34; P = 0.029). Nonetheless, their total relative risk of undergoing DBS treatment was 0.74 (95%CI, 0.48–1.12) compared to men with PD. At baseline, women had longer disease duration and worse dyskinesia. Exploring QoL domains, women reported worse mobility and bodily discomfort. At follow-up, all main outcomes improved equally in both genders. Our study provides evidence of a gender gap in DBS for PD. Women and men with PD have distinct preoperative nonmotor and motor profiles. We advocate that more focus should be directed toward the implementation of gender equity as both genders benefit from DBS with equal clinical efficacy. This study provides Class II evidence of beneficial effects of DBS in women with PD compared to male controls.
Similar content being viewed by others
Introduction
Deep brain stimulation (DBS) is an effective treatment in advanced Parkinson’s disease (PD) improving quality of life (QoL)1,2, motor3, and nonmotor symptoms (NMS)4,5,6. PD affects men more frequently than women with an overall prevalence gender-ratio of 1.48:1 (M:F)7,8,9. In advanced stages of PD, women are at higher risk than men to develop motor complications, such as dyskinesia or motor fluctuations and manifest different nonmotor profiles than men10,11. Previous studies have shown disparities in access to DBS between men and women as women are less likely to undergo DBS10,12,13, which is out of proportion to prevalence data8. However, it is unclear, at which key steps from referral through indication evaluation until surgical procedures the disadvantages arise for women with PD. Furthermore, little is known about gender-related differences in postsurgical outcomes or distinct nonmotor and motor profiles that could explain this ‘gender gap’. In particular, gender differences in nonmotor outcomes following DBS have not been systematically investigated yet. Therefore, this study examined (1) gender proportions at key steps from referral for indication evaluations until DBS surgery and (2) gender differences at preoperative baseline and in postoperative outcomes at 6-month follow-up with respect to QoL, nonmotor, and motor symptoms. We hypothesized that (1) the gender gap at these key steps cumulates to an overall disadvantage for women with PD and (2) that there are distinct nonmotor and motor profiles in men and women undergoing DBS and that nonetheless both genders postoperatively experience beneficial QoL, motor, and nonmotor effects.
Results
Gender ratio in Parkinson’s disease: from indication evaluation to deep brain stimulation surgery
In the cross-sectional cohort, 316 patients were referred for DBS indication evaluations at the University Hospital Cologne during the time period of January 2015 until September 2020 (see Table 1 and Fig. 1, women: n = 102). The gender ratio men:women was 2.1:1 (32% women). The proportion of women with PD referred for DBS indication evaluations was significantly lower than in the known general PD population of 1.48:17 (40% women; one-sample binomial test, P = 0.002), resulting in a 0.72 relative risk (RR, 0.72; 95%CI, 0.56–0.91) of referral for women compared to men with PD.
In (A), pie charts illustrate ratios of women (left) and men (right) with Parkinson’s disease who underwent DBS surgery, or did not undergo DBS surgery either despite positive indication evaluation or due to negative indication evaluation. In (B), bar charts illustrate the percentage of women and men with PD rejected for different reasons in DBS indication evaluations. 53 patients had one reason for rejection, 29 patients had at least two reasons for rejection, 5 patients had other reasons for rejection (mania, pre-existing orthopedic or cardiovascular conditions). The black star represents significantly more rejections due to depression in women than in men with PD. DBS Deep brain stimulation, PD Parkinson’s disease.
229 patients were approved for DBS in the multidisciplinary indication evaluations with a gender ratio of 1.8:1 (women n = 82). Of these, 190 patients underwent DBS surgery with a gender ratio of 2.0:1 (women n = 63). Female gender was associated with an approval decision for DBS, P = 0.029, the probability of approval was 17% higher in women with PD (RR, 1.17; 95%CI, 1.03–1.34).
Indication evaluations were negative in 20 women with PD and 67 men with PD (22% women). The main reasons for negative indication assessments were: clinically relevant neuropsychological impairment (n = 17, women: n = 2) and neuropsychiatric symptoms, such as depression (n = 27, women: n = 10), impulse control disorders not depending on dopaminergic medication (n = 25, women: n = 5), and hallucinations (n = 9, women: n = 0). Other reasons were an insufficient levodopa responsiveness (n = 19, women: n = 5) or a need for further medical optimization (n = 27, women: n = 6). Less frequent reasons for negative indications assessments were abuse disorders, mania, diagnosis of atypical PD, and a high risk for intraoperative bleedings because of previous illnesses. The proportion of rejections due to clinically relevant depression was higher for women with PD (P = 0.037). No gender differences were observed for other rejection reasons (all P > 0.05).
Among patients approved for DBS, the proportion of women who eventually underwent DBS surgery was smaller on trend level (RR, 0.89, m; 95%CI, 1.03–1.34; P = 0.065). The probability of undergoing DBS after positive indication evaluations was 11% lower in women with PD. The total relative risk of undergoing DBS treatment for women compared to men with PD was 0.73 (95%CI, 0.48–1.12, see Table 1). The reasons for not undergoing DBS surgery despite positive indication evaluations were: patient wish for an additional period of reflection (n = 10, women: n = 3), patient preference of further medical optimization (n = 8, women: n = 3), newly developed or worsened preexisting comorbid diseases (n = 9, women: n = 8), language barrier (n = 1, women: n = 1), and personal reasons undisclosed by patients (n = 11, women: n = 4). Chi-squared statistics showed no significant association between gender and these reasons.
DBS targeted the subthalamic nucleus in 157 patients (55 women), the internal segment of the globus pallidus in 18 patients (3 women), and the ventral intermediate nucleus of the thalamus in 15 patients (5 women). We observed no significant relationship between gender and DBS target (P > 0.05).
Clinical differences of men and women with Parkinson’s disease undergoing deep brain stimulation
Clinical assessments were conducted in a longitudinal multicenter cohort of 189 patients (women: n = 68, 36%). The mean age at preoperative baseline was 62.3 years ±8.5 and the mean time to follow-up was 0.5 ± 0.2 years.
Baseline characteristics of the original cohort
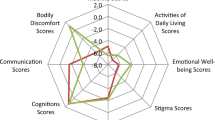
Women with PD had a longer disease duration (∆2.5 years; 95%CI, 1.0–4.0; P = 0.001; see Table 2) and more severe dyskinesia (∆13.1; 95%CI, 4.2–22.0; P = 0.001). No significant gender differences were observed for the PDQ-8 SI, NMSS total score, and LEDD. Exploring PDQ-8 and NMSS domains (see Fig. 2), we observed that women with PD experienced worse PDQ ‘mobility’ (∆0.3; 95%CI, 0.0–0.7; P = 0.044) and ‘bodily discomfort’ (∆0.6; 95%CI 0.2–0.9; P = 0.002), whereas men with PD experienced worse NMSS ‘sexual functions’ (women: median 0.0, IQR [interquartile range], 0.0–0.0; men: median 0.0, IQR 0.0–6.0; P = 0.001).
Figure 2 illustrates motor (left), nonmotor (middle), and quality of life (right) domains in women and men with Parkinson’s disease undergoing bilateral deep brain stimulation. NMSS Non-motor Symptom Scale, PD Parkinson’s disease, PDQ-8 Parkinson’s Disease Questionnaire-8, SCOPA-M Scales for Outcomes in Parkinson’s Disease-motor scale. Significant differences between women and men at preoperative baseline highlighted with: * for P < 0.05. ** for P < 0.01.
Clinical outcomes of the original cohort
Women and men with PD experienced improvements of the PDQ-8 SI, NMSS total score, and SCOPA-M total score (see Tables 3 and 4) and LEDD reductions. We observed no gender differences in these outcomes at 6-month follow-up in the between-group comparison (all P > 0.05). However, post-hoc exploratory analyses of domains of these scores revealed following differences: For the NMSS, beneficial effects were observed in both genders in the ‘sleep/fatigue’, ‘urinary’, and ‘miscellaneous’ domains. Only men with PD significantly improved in ‘mood/apathy’ and ‘perceptual problems/hallucinations’, whereas only women with PD experienced an improvement in ‘attention/memory’. In SCOPA-M subscores, we observed an improvement in both genders for ‘tremor’, ‘dyskinesia’, and ‘motor fluctuations’, whereas ‘bradykinesia’ improved only in men with PD. For the PDQ-8, we observed an improvement in both genders in the ‘mobility’, ‘activities of daily living’, ‘cognition’, ‘bodily discomfort’, and ‘stigma’ domains, whereas ‘emotional well-being’ improved only in men with PD.
The strength of clinical responses for women and men with PD and number needed to treat results are presented in Supplementary Tables 1 and 2. In summary, differences in effect sizes were small and favorable for men in emotional well-being, SCOPA-M total, bradykinesia, motor fluctuations, and LEDD and for women in attention/memory.
The matched sub-cohort: baseline characteristics and clinical outcomes
Propensity score matching resulted in a sub-cohort of 116 patients (58 women and 58 men). Balance diagnostics indicated a good matching between the two groups with no significant differences for all main demographic and clinical outcome parameters. Baseline characteristics of the matched sub-cohort are reported in Supplementary Table 3 and clinical outcomes in Supplementary Tables 4 and 5. No significant gender differences were observed for all main outcomes at 6-month follow-up. The clinical outcomes of the matched sub-cohort did not differ from the original cohort.
Discussion
Our study provides evidence of a gender gap in DBS for PD: (1) Disproportionally fewer women underwent DBS indication assessments than to be expected from the gender ratio of the general PD population, (2) preoperatively, mean PD duration was longer and dyskinesia more severe in women with PD, and, (3) nonetheless, DBS was equally clinically efficacious on total QoL, nonmotor, and motor symptoms burden in women and men with PD (Class II evidence).
In our cross-sectional cohort, disproportionally fewer women were referred for DBS indication evaluations as the gender ratio was (men:women) 2.1:1 as compared to the gender proportion in the known PD population 1.48:17,14. In women, 80% of referred patients were approved for DBS, which was significantly higher than the 69% approval rate in men.
The observation that indication evaluations were negative in only 20% of women compared to 31% in men, however does not indicate that the gender effect is beneficial for women with PD as women experienced more severe preoperative motor complications10. This bias represents not a local, but a systematic effect and has also been observed in other cohorts in the USA (Miami8 and Medicare Services12) and Europe (Düsseldorf15 and Umeå16). Previous studies indicate that the gender gap in assessments of eligibility for DBS may result from nonclinical factors15,17 and possible explanations include gender referral biases to specialty care18,19, patient preferences regarding medical care8 including greater fear of surgery among women20, and unmeasured clinical characteristics, such as socioeconomic status18. The proportion of rejections due to clinically relevant depression was higher for women with PD. Rejections were based on assessments in multi-disciplinary team meetings in which patients also participated. In these meetings, patients’ history of affective and neurological symptoms, evaluations of expert psychiatrists experienced in DBS indication evaluations for PD, and neuropsychological depression test scores were taken into consideration. As previous studies show that <30% of PD patients consent to referral for DBS evaluations, further research is needed regarding the referral processes of general practitioners and neurologists21. Gender ratios in DBS cohorts seem to align better with the PD prevalence when patients receive specially developed educational material and referring medical professionals use DBS screening tools8,15. In our study, the odds of undergoing DBS surgery after being evaluated as a good candidate for DBS was ~27% lower in women than in men with PD. To our knowledge, our study is the first to report gender ratios at key steps from referral through indication evaluations until DBS surgery. Further studies are needed to investigate why women with PD approved for DBS treatment do not undergo DBS surgery eventually.
Looking beyond DBS cohorts, women are also underrepresented for invasive treatments in other diseases, such as cardiac22,23 or gastrointestinal conditions24. Future studies in gender medicine are needed to investigate the deliberation process of patients and the clinical reasoning of referring medical professionals and of hospital staff in which patients undergo invasive treatments. These studies should focus on the decisional process rather than the decision’s end results, which may help to develop new approaches to understand and influence these gender disparities.
In our longitudinal original cohort, in line with previous DBS studies for PD, disease duration was longer in women with PD8. This might be explained by the fact that women appear to have slower disease progression25. Confirming results of previous studies, women reported more severe motor complications than men before undergoing DBS surgery, which mainly resulted from dyskinesia16,26. In line with a study by Hariz et al., we did not observe significant gender differences in total preoperative QoL and women specifically reported worse bodily discomfort and mobility16. We observed no preoperative gender differences in overall NMS burden. Confirming previous studies27, we observed no gender differences in mood/apathy and attention/memory. Men reported worse preoperative sexual functions than women which is in line with previous research28.
In line with previous studies, we observed similar improvements of total QoL and motor outcomes in women and men with PD undergoing DBS in our original cohort16,29,30. Our study expanded on the existing literature by systematically comparing gender differences in nonmotor effects of DBS for PD. We observed similar beneficial effects in both genders on total NMS burden. However, we found distinct DBS effect profiles for specific NMS in women and men with PD: Only women experienced an improvement in ‘attention/memory’, whereas only men experienced beneficial effects in the ‘mood/apathy’ and ‘perceptual problems/hallucinations’ domains. Further studies are needed to investigate possible reasons for differential effects on specific NMS in women and men with PD, in particular, considering gender differences in brain structure and function in PD31.
In summary, our study presents evidence that different stakeholders may contribute to the gender gap observed in DBS: (1) general practitioners and neurologists refer disproportionally fewer women than men for DBS indication evaluations, (2) women with PD with positive indication evaluations undergo DBS surgery less likely than men with PD, to which (3) hospital medical staff may contribute as all indication assessments are conducted in the setting of in-patient care which provides ample time to convey the rationale and clinical reasoning for a treatment with DBS when indication evaluations are positive. Monitoring gender ratios in DBS is informative, but this does not address the underlying reasons for gender disparities outlined here. Closely connected to this point, the gender gap is still evident despite the implementation of ‘gender mainstreaming strategies’ in healthcare systems around the globe32. Therefore, we advocate that more focus should be directed toward the decisional processes and the responsibilities of stakeholders in the implementation of gender equity in the context of DBS treatment. A pioneer interview study included 11 women with PD but lacked clinical data to investigate how patients’ nonmotor or motor symptom profiles influence decision-making processes33.
The present work has limitations. In the cross-sectional cohort, the reasons why patients did not receive DBS were analyzed retrospectively and the reasons of DBS referrals were not assessed systematically. Therefore, this study does not consider the number of patients who declined referral for DBS evaluations. Further studies including surveys in referring general practitioners and neurologists are needed. In the longitudinal cohort, although the cohort size of 189 patients was one of the biggest in studies of its kind, especially the group of women with PD (n = 68) was relatively small. As this was a “real-world study” we did not examine motor OFF states and all assessments were conducted in clinical ON states. However, NMS and QoL were surveyed over the previous 4 weeks and, therefore, reflect ON and OFF states. Clinical ratings were performed by unblinded raters. However, raters were unaware of the research question regarding gender difference analyses, so a bias resulting from a lack of blinding is improbable. As this was a “real-world study”, we used abbreviated QoL and motor scales (PDQ-8 and SCOPA-motor scale) which highly correlate with the scales from which they were derived (PDQ-39 and UPDRS-III). However, the use of the latter scales may have revealed small differences amongst women and men with PD better due to their finer gradation. Furthermore, minimal clinically important changes have not been published for the NMSS yet. Therefore, the clinical relevance of our results was assessed based on Cohen’s d effect sizes34. In our cohort, baseline disease duration was longer and dyskinesia more severe in women with PD. Therefore, we used propensity scores to identify a sub-cohort which was precisely matched for these variables and, thereby, establish a quasi-experimental design to confirm results of the original cohort. While propensity score matching has advantages as a method providing a ‘pseudo-randomization’ in observational studies, it cannot replace a randomized clinical trial. However, in certain scenarios, such as in our database, the real-life presentation of women and men with PD may be of scientific interest. Here, propensity score matching provides an accurate approach to increase causal inference. An inclusion of demographic and clinical parameters in the matching procedure and an implementation of strict comprehensive diagnostic statistics increase the validity of our results. However, this method can only be applied to parameters assessed clinically. Therefore, it should be noted, that there are other contributors to DBS outcomes beyond the factors, which are considered in the propensity score matching, and that this method does not consider potentially relevant parameters, which were not measured, for example impulse control disorders. Acknowledging the possibility of unknown confounders, we used independent samples tests for all further statistical tests for comparisons between women and men with PD35. Furthermore, one has to acknowledge that DBS cohorts are highly selected and that dementia and severe depression are considered to be contraindications for DBS. Therefore, our results cannot be generalized to PD patients with severe impairments in these NMS.
Another limitation of this study is the short time period of 6 months for the evaluation of the outcome of DBS in PD patients. However, a similar improvement of both genders was also observed in a study, which analyzed gender differences regarding motor function, dyskinesia, the 36-item Short Form Health Survey, the Mini-Mental State Examination, and the Beck’s Depression Inventory 5 years after STN-DBS27. In this study, Kim et al. reported favorable long-term effects of DBS in men on QoL preservation. Further studies are needed to investigate gender differences of nonmotor long-term effects of DBS beyond depression and global cognition.
We observed a gender gap in patients undergoing DBS indication evaluations and treatment. The reasons for this gender gap seem to be nonclinical as the proportion of women with PD with positive indication evaluations who eventually underwent DBS was lower than in men with PD even though DBS efficacy was equal regarding total QoL, nonmotor, and motor symptoms. Therefore, to implement gender equity, we propose that more focus should be spent on nonclinical factors, such as deliberation processes of women with PD and clinical reasoning of referring general practitioners and neurologists. The observation of distinct effect profiles in women and men with PD for specific NMS highlights the need of holistic assessments of nonmotor and motor symptoms in patients with PD undergoing DBS. Therefore, in accordance with the concept of personalized medicine, we advocate considering nonclinical parameters and the evaluation of holistic clinical assessments side-by-side to tailor PD treatment to patients’ individual needs36.
Methods
Study design and ethical approval
We analyzed longitudinal data from the prospective, observational, multicenter international NILS study37 including centers in Cologne, Marburg, Greater Manchester, and London. All patients gave written informed consent before study procedures. The study was performed in accordance with the Declaration of Helsinki (German ClinicalTrials Register DRKS00006735, local ethics committees master votes for Germany: Cologne #12/145, and for UK: NRES South-East London REC3-10/H0808/141 #10084).
In addition, we conducted a retrospective chart review of referrals and indication evaluations for DBS in a cross-sectional cohort of the University Hospital Cologne from January 2015 to September 2020.
Participants
PD diagnosis was based on the British Brain Bank criteria38 in women and men. Patients were screened for DBS according to Movement Disorders Society (MDS) guidelines39. DBS surgery was considered when levodopa responsiveness was sufficient (>30% improvement in the Unified PD Rating Scale-motor examination, UPDRS-III). Patients were not eligible for DBS treatment if they suffered from clinically relevant psychiatric diseases or neuropsychological impairments40 as assessed by a multidisciplinary team of specialized neurologists, neuropsychologists, stereotactic neurosurgeons, psychiatrists, speech and physiotherapists. In the longitudinal cohort, all patients received bilateral STN-DBS. In the cross-sectional cohort, patients undergoing DBS were implanted in the STN, globus pallidus internus or ventral intermediate nucleus.
Clinical assessment
Patients were assessed in the ON-medication state (MedON) at preoperative baseline and in a clinical medication and stimulation ON state (MedON/StimON) at postoperative 6-month follow-up. The following scales were assessed:
QoL was assessed with the PD Questionnaire-8 (PDQ-8) which covers eight QoL domains (mobility, activities of daily living, emotional well-being, social support, cognition, communication, bodily discomfort, and stigma)41. It is recommended by the MDS Scales Committee for QoL assessments42 and has been used in DBS studies43,44,45,46. The results are presented as PDQ-8 Summary Index (PDQ-8 SI).
NMS were assessed with the NMSScale (NMSS)47. The scale consists of 30 items for nine nonmotor domains of PD (cardiovascular, sleep/fatigue, mood/apathy, perceptual problems/hallucinations, attention/memory, gastrointestinal symptoms, urinary symptoms, sexual function, and miscellaneous symptoms).
Motor disorder was assessed with the Scales for Outcomes in PD-motor scale (SCOPA-M). The SCOPA-M is a modified version of the UPDRS48, strongly correlates with the corresponding subscales of the UPDRS, and has been used in DBS studies before37,49,50. The SCOPA-M was preferred because its assessment time is four times shorter than the MDS-UPDRS51. A study by Rooden et al.52 combined items from the motor examination and activities of daily living sections of the SCOPA-M and, using a data-driven approach, identified the following motor aspects in an exploratory factor analysis: (1) axial (postural and locomotor) symptoms, (2) axial (general) symptoms, such as speech and swallowing, and ‘freezing during on’, (3) tremor, and (4) bradykinesia and rigidity. As published previously by our group4, to better distinguish between these axial symptoms, we included only speech and swallowing in a subscore for ‘dysphagia and dysarthria’ and report subscores for ‘dyskinesia’ and ‘motor fluctuations’.
The levodopa equivalent daily dose (LEDD) was calculated according to the method by Tomlinson et al.53.
Statistical analysis
In the cross-sectional cohort, to analyze clinical practice of referrals and DBS indication assessments for women and men with PD, we conducted a systematic chart review for consecutive patients at the University Hospital Cologne between January 2015 and September 2020. We recorded the total number of referrals and positive and negative indication assessments in women and men with PD. A one-sample binomial test was employed to compare the ratio of women with PD referred for DBS indication to the ratio of women in the general PD population. Further, we analyzed differences in gender proportions using Chi-square tests or Fisher’s exact test, when sample size was low (expected values in any of the cells of a contingency table below 5) at the following key steps: (1) referrals for DBS indication evaluation, (2) positive and negative decisions of indication evaluations, (3) reasons for negative DBS evaluations, and (4) DBS surgery. We calculated relative risk statistics for women compared to men with PD at these steps. The term ‘relative risk’ is used in the statistical sense for the ratio of the probabilities of a certain outcome in two groups, whether the outcome is medically favorable (e.g., treatment with DBS) or unfavorable (e.g., development of complications). To compare the total relative risk for DBS treatment of women compared to men with PD, we multiplied the relative risks of referral with those of following steps (positive indication evaluations and DBS surgery).
In the longitudinal cohort, we analyzed clinical outcomes of women and men with PD. Normal distribution was tested with the Shapiro–Wilk test. Preoperative gender differences were analyzed using Mann–Whitney U tests or unpaired t-tests when parametric test criteria were fulfilled. Within-group changes at 6-month follow-up were analyzed with Wilcoxon signed-rank or paired samples t-tests, when parametric test criteria were fulfilled. Change scores (testbaseline − testfollow-up) were calculated to compare differences between men and women using Mann–Whitney U or unpaired t-tests. In addition, we computed relative changes ([testbaseline − testfollow-up]/testbaseline) and Cohen’s effect sizes including confidence intervals based on non-central t distribution according to a method by Smithson54. Furthermore, we calculated number needed to treat (1/% of patients improving > ½ SD testbaseline pooled). Multiple comparisons due to multiple outcome parameters were corrected with the Benjamini–Hochberg method. All P values presented are adjusted to the P < 0.05 significance threshold. Post-hoc, we explored PDQ-8, NMSS, and SCOPA-M domain outcomes. In addition, to account for potential baseline differences between women and men with PD, we used Propensity Score Matching for SPSS (version 3.04)55. Matching variables were age at intervention, disease duration, and baseline SCOPA-dyskinesia. We implemented nearest-neighbor matching with a 0.25 caliper56 without replacement employing a 1:1 ratio (women:men). Balance diagnostics were conducted based on Cohen’s effect size |d | <0.2556. Subsequently, all analyses of clinical changes were also carried out for the thus identified matched sub-cohort.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
Statistical analyses were performed using SPSS Statistics 25. SPSS codes are available upon reasonable request to the corresponding authors.
References
Schuepbach, W. M. et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 368, 610–622 (2013).
Jost, S. T. et al. Non-motor predictors of 36-month quality of life after subthalamic stimulation in Parkinson disease. NPJ Parkinsons Dis. 7, 48 (2021).
Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355, 896–908 (2006).
Jost, S. T. et al. A prospective, controlled study of non-motor effects of subthalamic stimulation in Parkinson’s disease: results at the 36-month follow-up. J. Neurol. Neurosurg. Psychiatry 91, 687–694 (2020).
Dafsari, H. S. et al. Beneficial effect of 24-month bilateral subthalamic stimulation on quality of sleep in Parkinson’s disease. J. Neurol. 267, 1830–1841 (2020).
Anna, S. P. et al. The New Satisfaction with Life and Treatment Scale (SLTS-7) in Patients with Parkinson’s Disease. J. Parkinson Dis. 12, 453–464 (2022).
Moisan, F. et al. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J. Neurol. Neurosurg. Psychiatry 87, 952–957 (2016).
Shpiner, D. S. et al. Gender Disparities in Deep Brain Stimulation for Parkinson’s Disease. Neuromodulation 22, 484–488 (2019).
Katz, M., Kilbane, C., Rosengard, J., Alterman, R. L. & Tagliati, M. Referring patients for deep brain stimulation: an improving practice. Arch. Neurol. 68, 1027–1032 (2011).
Picillo, M. et al. The relevance of gender in Parkinson’s disease: a review. J. Neurol. 264, 1583–1607 (2017).
Martinez-Martin, P. et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J. Neurol. https://doi.org/10.1007/s00415-011-6392-3 (2012).
Willis, A. W. et al. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 82, 163–171 (2014).
Hariz, G. M. et al. Gender distribution of patients with Parkinson’s disease treated with subthalamic deep brain stimulation; a review of the 2000-2009 literature. Parkinsonism Relat. Disord. 17, 146–149 (2011).
Meoni, S., Macerollo, A. & Moro, E. Sex differences in movement disorders. Nat. Rev. Neurol. 16, 84–96 (2020).
Dinkelbach, L., Möller, B., Witt, K., Schnitzler, A. & Südmeyer, M. How to improve patient education on deep brain stimulation in Parkinson’s disease: the CARE Monitor study. BMC Neurol. 17, 36 (2017).
Hariz, G. M. et al. Gender differences in quality of life following subthalamic stimulation for Parkinson’s disease. Acta Neurol. Scand. 128, 281–285 (2013).
Morgante, L. et al. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat. Disord. 13, 528–531 (2007).
Bhave, P. D., Lu, X., Girotra, S., Kamel, H. & Vaughan Sarrazin, M. S. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm 12, 1406–1412 (2015).
Feldman, D. E. et al. Gender and other disparities in referral to specialized heart failure clinics following emergency department visits. J. Women’s Health (Larchmt.) 22, 526–531 (2013).
Setiawan, M. et al. Referrals for movement disorder surgery: under-representation of females and reasons for refusal. Can. J. Neurol. Sci. 33, 53–57 (2006).
Wächter, T., Mínguez-Castellanos, A., Valldeoriola, F., Herzog, J. & Stoevelaar, H. A tool to improve pre-selection for deep brain stimulation in patients with Parkinson’s disease. J. Neurol. 258, 641–646 (2011).
Chibber, T. & Baranchuk, A. Sex-Related Differences in Catheter Ablation for Patients With Atrial Fibrillation and Heart Failure. Front. Cardiovasc. Med. 7, https://doi.org/10.3389/fcvm.2020.614031 (2020).
Hvelplund, A. et al. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur. Heart J. 31, 684–690 (2010).
Herold, A. H. et al. Evidence of gender bias in patients undergoing flexible sigmoidoscopy. Cancer Detect Prev. 21, 141–147 (1997).
Haaxma, C. A. et al. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 78, 819–824 (2007).
Accolla, E. et al. Gender differences in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Mov. Disord. 22, 1150–1156 (2007).
Kim, R. et al. Sex differences in the short-term and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Parkinsonism Relat. Disord. 68, 73–78 (2019).
Hand, A., Gray, W. K., Chandler, B. J. & Walker, R. W. Sexual and relationship dysfunction in people with Parkinson’s disease. Parkinsonism Relat. Disord. 16, 172–176 (2010).
Romito, L. M., Contarino, F. M. & Albanese, A. Transient gender-related effects in Parkinson’s disease patients with subthalamic stimulation. J. Neurol. 257, 603–608 (2010).
Chandran, S. et al. Gender influence on selection and outcome of deep brain stimulation for Parkinson’s disease. Ann. Indian Acad. Neurol. 17, 66–70 (2014).
Tremblay, C. et al. Sex effects on brain structure in de novo Parkinson’s disease: a multimodal neuroimaging study. Brain 143, 3052–3066 (2020).
Gupta, G. R. et al. Gender equality and gender norms: framing the opportunities for health. Lancet 393, 2550–2562 (2019).
Hamberg, K. & Hariz, G. M. The decision-making process leading to deep brain stimulation in men and women with parkinson’s disease - an interview study. BMC Neurol. 14, 89 (2014).
Dafsari, H. S. et al. Beneficial nonmotor effects of subthalamic and pallidal neurostimulation in Parkinson’s disease. Brain Stimul. 13, 1697–1705 (2020).
Williamson, E., Morley, R., Lucas, A. & Carpenter, J. Propensity scores: from naive enthusiasm to intuitive understanding. Stat. Methods Med. Res. 21, 273–293 (2012).
Valentina, L. et al. Personalised Advanced Therapies in Parkinson’s Disease: The Role of Non-Motor Symptoms Profile. J. Pers. Med. 11, 773 (2021).
Jost, S. T. et al. Subthalamic stimulation improves quality of sleep in parkinson disease: a 36-month controlled study. J. Parkinsons Dis. 11, 323–335 (2021).
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184 (1992).
Lang, A. E. et al. Deep brain stimulation: preoperative issues. Mov. Disord. 21, S171–S196 (2006). Suppl 14.
Florin, E. et al. Modulation of local field potential power of the subthalamic nucleus during isometric force generation in patients with Parkinson’s disease. Neuroscience 240, 106–116 (2013).
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R. & Hyman, N. The PDQ-8: development and validation of a short-form Parkinson’s Disease Questionnaire. Psychol. Health 12, 805–814 (1997).
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M., Chaudhuri, K. R. & Group, N. V. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 26, 399–406 (2011).
Storch, A. et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80, 800–809 (2013).
Salimi, H. et al. Subthalamic Stimulation Improves Quality of Life of Patients Aged 61 Years or Older With Short Duration of Parkinson’s Disease. Neuromodulation Technol. Neural Interface 21, 532–540 (2018).
Claudia, L-O. et al. Evaluation of the effect of bilateral subthalamic nucleus deep brain stimulation on fatigue in Parkinson’s Disease as measured by the non-motor symptoms scale. Br. J. Neurosurg. 1–4, https://doi.org/10.1080/02688697.2021.1961681 (2021).
Anna, S. Predictors of short-term impulsive and compulsive behaviour after subthalamic stimulation in Parkinson disease. J. Neurol. Neurosurgery Psychiatry 92, 1313–1318 (2021).
Chaudhuri, K. R. et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 22, 1901–1911 (2007).
Marinus, J. et al. A short scale for the assessment of motor impairments and disabilities in Parkinson’s disease: the SPES/SCOPA. J. Neurol. Neurosurg. Psychiatry 75, 388–395 (2004).
Sauerbier, A. et al. Clinical Non-Motor Phenotyping of Black and Asian Minority Ethnic Compared to White Individuals with Parkinson’s Disease Living in the United Kingdom. J. Parkinsons. Dis. https://doi.org/10.3233/JPD-202218 (2020).
Dafsari, H. S. et al. Beneficial effects of bilateral subthalamic stimulation on alexithymia in Parkinson’s disease. Eur. J. Neurol. 26, 222–e217 (2019).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
van Rooden, S. M., Visser, M., Verbaan, D., Marinus, J. & van Hilten, J. J. Motor patterns in Parkinson’s disease: a data-driven approach. Mov. Disord. 24, 1042–1047 (2009).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Smithson, M. Confidence intervals. (Sage, 2003).
Thoemmes, F. Propensity Score Matching in SPSS. arXiv:1201.6385 (2012).
Stuart, E. A. & Rubin, D. B.Best practices in quasi-experimental designs. (Sage Publications, 2008)
Acknowledgements
The authors wish to thank their patients for participating in this study. The London center (KRC, AR, KA) wishes to thank Dr. M. Samuel for attending to patients treated with deep brain stimulation in clinical routine and also the BRC for funding the NILS database. The Marburg center (LT, PAL, CN) wishes to thank Dr. D. Pedrosa for allocating patients to specific studies and attending to patients treated with deep brain stimulation in clinical routine.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
S.T.J.—data acquisition, statistical analyses of longitudinal data, drafting of the paper. L.S.—data acquisition, data analysis, drafting of the paper. A.R.—data acquisition, critical revision of the paper. P.A.L.—data acquisition, critical revision of the paper. K.A.—surgical procedures, critical revision of the paper. J.E.—surgical procedures, critical revision of the paper. F.R.—data acquisition, critical revision of the paper. M.T.B.—critical revision of the paper. G.R.F.—critical revision of the paper. J.F.—statistical analysis of cross-sectional data, critical revision of the paper. A.S.—data acquisition, critical revision of the paper. C.N.—surgical procedures, critical revision of the paper. A.S.—critical revision of the paper. K.R.C.—study concept and design, data acquisition, critical revision of the paper. A.A.—critical revision of the paper. L.T.—study concept and design, critical revision of the paper. P.M.M.—study concept and design, critical revision of the paper. M.S.—data acquisition, critical revision of the paper. E.K.—critical revision of the paper. V.V.V.—surgical procedures, critical revision of the paper. H.S.D.—study concept and design, data acquisition, data analysis, drafting of the paper, critical revision of the paper. S.T.J. and L.S. contributed equally to this paper and are considered co-first authors.
Corresponding authors
Ethics declarations
Competing interests
S.T.J. was funded by the Prof. Klaus Thiemann Foundation. L.S. reports no financial disclosures. A.R. has received honorarium from UCB and was supported by a grant from Medtronic. P.A.L. was funded by the SUCCESS-Program of the University of Marburg, the Parkinson’s Foundation, and the Stiftung zur Förderung junger Neurowissenschaftler. K.A. has received honoraria for educational meetings, travel and consultancy from Medtronic, St. Jude Medical and Boston Scientific. J.E. reports no financial disclosures. F.R. reports no financial disclosures. M.T.B. received speaker’s honoraria from Medtronic, Boston Scientific, Abbott (formerly St. Jude), GE Medical, UCB, Apothekerverband Köln e.V. and Bial as well as research funding from the Felgenhauer-Stiftung, Forschungspool Klinische Studien (University of Cologne), Horizon 2020 (Gondola), Medtronic (ODIS), and Boston Scientific and advisory honoraria for the IQWIG. G.R.F. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 431549029—SFB 1451. G.R.F. serves as an editorial board member of Cortex, Neurological Research and Practice, NeuroImage: Clinical, Zeitschrift für Neuropsychologie, and DGNeurologie; receives royalties from the publication of the books Funktionelle MRT in Psychiatrie und Neurologie, Neurologische Differentialdiagnose, and SOP Neurologie; receives royalties from the publication of the neuropsychological tests KAS and Köpps; received honoraria for speaking engagements from Bayer, Desitin, DGN, Ergo DKV, Forum für medizinische Fortbildung FomF GmbH, GSK, Medica Academy Messe Düsseldorf, Medicbrain Healthcare, Novartis, Pfizer, and Sportärztebund NRW. Jeremy Franklin reports no financial disclosures. A.S. is funded by the Gusyk program and the Advanced Cologne Clinician Scientist program of the Medical Faculty of the University of Cologne and has received funding from the Prof. Klaus Thiemann Foundation. C.N. is consultant for Brainlab and received speaker’s honoraria. A.S. reports no financial disclosures. K.R.C. has received funding from Parkinson’s UK, NIHR, UCB, and the European Union; he received honoraria from UCB, Abbott, Britannia, US Worldmeds, and Otsuka Pharmaceuticals; and acted as a consultant for AbbVie, UCB, and Britannia. A.A. reports personal consultancy fees from Zambon, AbbVie, Boehringer Ingelheim, GE, Neuroderm, Biogen, Bial, EVER Neuro Pharma, Therevance, Vectura grants from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020 - PD_Pal Grant 825785, Ministry of Education University and Research (MIUR) Grant ARS01_01081, owns Patent WO2015110261-A1, owns shares from PD Neurotechnology Limited. L.T. reports grants, personal fees and non-financial support from SAPIENS Steering Brain Stimulation, Medtronic, Boston Scientific and St. Jude Medical. P.M-M. has received honoraria from National School of Public Health (ISCIII), Editorial Viguera and Takeda Pharmaceuticals for lecturing in courses; from Britannia for writing an article in their Parkinson’s Disease Medical Journal-Kinetic; and from the International Parkinson and Movement Disorder Society (MDS) for management of the Program on Rating Scales. Grants: from the MDS for development and validation of the MDS-NMS. Monty Silverdale has received honoraria from Bial, Britannia and Medtronic. E.K. has received grants from the German Ministry of Education and Research, the German Parkinson- Fonds, the German Parkinson Society; honoraria from: Oticon GmbH, Hamburg, Germany; Lilly Pharma GmbH, Bad Homburg, Germany; Bernafon AG, Bern, Switzerland; Desitin GmbH, Hamburg, Germany. V.V.V. is a member of the advisory boards and reports consultancies for Medtronic, Boston Scientific and St. Jude Medical. She received a grant from SAPIENS Steering Brain Stimulation. H.S.D. reports funding of his work by the EU Joint Programme—Neurodegenerative Disease Research (JPND), the Prof. Klaus Thiemann Foundation, the Felgenhauer Foundation, and the Koeln Fortune Program, and honoraria by Boston Scientific, Medtronic and Stadapharm. This paper presents independent research funded by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Unit at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jost, S.T., Strobel, L., Rizos, A. et al. Gender gap in deep brain stimulation for Parkinson’s disease. npj Parkinsons Dis. 8, 47 (2022). https://doi.org/10.1038/s41531-022-00305-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00305-y
This article is cited by
-
Architecture of the subthalamic nucleus
Communications Biology (2024)
-
Access to device-aided therapies in advanced Parkinson’s disease: navigating clinician biases, patient preference, and prognostic uncertainty
Journal of Neural Transmission (2023)
-
Tools and criteria to select patients with advanced Parkinson’s disease for device-aided therapies: a narrative review
Journal of Neural Transmission (2023)
-
Adaptive deep brain stimulation for Parkinson’s disease: looking back at the past decade on motor outcomes
Journal of Neurology (2023)
-
Gender-specific outcomes of deep brain stimulation for Parkinson’s disease — results from a single movement disorder center
Neurological Sciences (2023)