Abstract
Safinamide is a highly selective, reversible MAO B-inhibitor recently marketed in European and North American countries. To better define clinical indications regarding motor and non-motor symptoms, targeted population and safety of this compound, ten movement disorders specialists, experts in their field, convened and developed a panel of statements on: the role of glutamate in Parkinson’s disease, introduction to fluctuations, efficacy of safinamide on motor symptoms, motor complications and non-motor symptoms, quality of life, safety of safinamide and target population for use. Strong consensus was reached for all the statements on the efficacy of safinamide on motor symptoms, motor fluctuations, quality of life and safety. Among non-motor symptoms, a positive consensus was reached for the symptoms sleep/fatigue, mood, and pain while there was a lack of consensus for the statements regarding the efficacy of safinamide in improving cognition, urinary and sexual functions. The statement on orthostatic hypotension obtained a negative consensus. The consistent and large agreement reached in this Delphi panel perfectly reflects the perception of efficacy, safety and tolerability of safinamide as evident from pivotal trials and clinical practice and shows how these findings may guide movement disorders specialists in their clinical therapeutic approach. The impact of non-motor symptoms in PD is considerable, and management remains an unmet need. In this context, the ability of safinamide to impact some non-motor symptoms may represent the most promising and distinctive feature of this compound and deserves further investigations.
Similar content being viewed by others
Introduction
Monoamine oxidase B inhibitors represent an important treatment option in the management of Parkinson’s disease (PD), both in the early and in the advanced stages of motor complications1,2. The clinical benefit of MAO-B inhibitors arises from the ability of these medications to enhance the level of dopamine by decreasing its catabolism in the brain.3. Among MAO-B inhibitors, safinamide has a dual-mechanism of action since it is able to inhibit (a) MAO-B, potentiating dopaminergic transmission and (b) glutamate release by blocking voltage-dependent sodium channels and modulating calcium channels4,5. Safinamide is a benzylamine derivative which acts as potent, highly selective, and reversible MAO-B inhibitor. Due to its reversibility4,6, treatment with safinamide is associated with reduced risk of hypertensive crises or serotonergic syndrome7,8 as well as drug interaction4,6.
Safinamide is marketed in Europe and in North America under the brand name of Xadago® (Zambon Pharma) and Onstryv® (Valeo Pharma); it was approved in 2015 as adjunctive therapy to levodopa in mid- to late-stage fluctuating patients and became commercially available in the spring of 2016. While its efficacy in controlling motor symptoms and improving motor fluctuations is well established9,10,11, uncertainty remains on its potential ability to control dyskinesias in the long term or to address the many non-motor symptoms that are typical of the advanced stages of the disease. The aim of this study was to obtain a European Consensus on the use of safinamide, considering the efficacy of this compound on motor symptoms and motor complications, its effect on non-motor symptoms (NMS), quality of life in patient with PD and how its clinical effect is perceived by clinicians. Moreover, we wished to identify an ideal target population and delineate the safety in different PD patient sub-populations.
Results
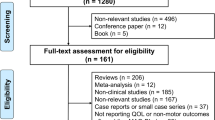
Hundred and nineteen panelists among movement disorders specialists were identified in the following countries: Italy, UK, Belgium, Spain, Germany, Sweden, Austria, Netherlands. The response rate was 76% (n = 90) which was considered high, taking into account that the study was performed during the COVID 19 pandemic and related lockdown in most countries.
Strong consensus was reached for all the statements regarding the efficacy of safinamide on motor symptoms, motor fluctuations, quality of life and safety (29/34 statements, 85,2 % of agreement, mean score 91.5), suggesting a shared view of European movement disorders specialists on these topics. Among NMS, a common, positive consensus was achieved for the symptoms sleep/fatigue, mood, and pain while there was a lack of consensus for the statements regarding the efficacy of safinamide in improving cognition, urinary and sexual functions. No agreement was reached either on the tolerability of safinamide in patients with hallucinations. The statement on orthostatic hypotension obtained a negative consensus.
A second round of questionnaire was deemed unnecessary by the Board, since all the statements with no or negative agreement were considered clearly stated and results obtained reflected trends in clinical practice.
Table 1 summarizes the statements and the percentage of agreement/disagreement reached, based on the responses of the 90 Panelists.
Topic 1: Glutamate role in Parkinson’s disease
Statements 1.1–1.8 (Fig. 1): the glutamatergic circuits within the basal ganglia were confirmed to have a relevant role in the normal control of movement (96% agreement), pain, mood, and cognition (93%). A large consensus was reached on the statement that the glutamatergic pathway is involved in the aetiopathogenesis of the disease (96%) and that the abnormal firing across the glutamatergic cortico-striatal pathway is a key mechanism in the development of dyskinesia (98%). Panelists´ opinions on the contributing role of glutamate in the emergence of motor symptoms of PD and in executive dysfunctions were less convergent, even if consensus was obtained for both statements (80 and 86%). Glutamate was considered to be involved in neuropathic pain (93%) but to be less crucial in the development of inflammatory pain (80%).
These statements reflect the high level of scientific evidence which has confirmed and defined the role of glutamate in motor and non-motor symptoms of PD12,13.
Topic 2: Introduction on fluctuations
Statements 2.1–2.3 (Fig. 2): the Panelists agreed that an early fluctuator can be defined as a patient who had motor fluctuations for no more than one year and that wearing off phenomena can be present in subjects taking no more than three doses of LD a day (99%). Ninety three percent of the Panelists found the use of WOQ19 and WOQ9 to be useful in the diagnosis of wearing off, in agreement with existing literature14,15.
Topic 3: Efficacy - Motor symptoms
Statement 3.2 (Fig. 3): there was almost unanimous consensus on the ability of safinamide to improve motor symptoms, both in the short and long term (98%).
Topic 3: Efficacy - Motor fluctuations
Statements 3.1 and 3.3–3.4 (Fig. 3): There was full agreement on the efficacy of safinamide in reducing off time (100%). The efficacy of safinamide was believed to be related not only to MAO-B inhibition (98%) and that the glutamate-modulating component of this compound contributes to its clinical efficacy in increasing good on time (without troublesome dyskinesia, 94%).
Topic 3: Efficacy - NMS
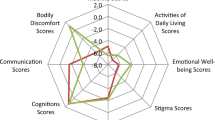
Statements 3.5–3.11 (Fig. 3): a positive consensus was achieved for the symptoms sleep/fatigue, mood, and pain (respectively 80, 83 and 81%) while no agreement was observed in relation to cognition, urinary and sexual function. Seventy-two percent of the Panelists disagreed with the statement: safinamide improves orthostatic hypotension.
Topic 4: Quality of life
Statement 4.1 (Fig. 4): The positive effect of safinamide on quality of life reached an almost unanimous consensus (98%).
Topic 5: Safety
Statements 5.1–5.8 (Fig. 5): the totality of the Panelists agreed with the statement that safinamide is safe as add-on therapy for symptomatic PD treatment and that its reversible inhibitory effect of MAO-B represents an advantage in clinical practice. Ninety-six percent of voters believed that safinamide could ameliorate on time without increasing troublesome dyskinesia but only 73% were convinced that the molecule is able to improve dyskinesia in the long-term. Agreement was reached regarding the safety of safinamide as an adjunct therapy in patients aged ≥75 years (95%) and in patients with cognitive impairment (89%). No consensus was reached regarding the tolerability of safinamide in patients with hallucinations (39 vs 61%).
Topic 5: Safety - Dyskinesia
Statement 5.3 (Fig. 5): The long-term efficacy of safinamide on dyskinesia reached a relatively weak agreement (73%), reflecting the conflicting results available from the literature to date.
Topic 6- Target population
Statements 6.1–6.3 (Fig. 6): safinamide was considered a valid therapeutic option in patients with advanced PD (100% agreement), in the early stages of motor fluctuations (97%) and as add-on to LD therapy in PD (100%).
Discussion
The results of this Delphi survey suggest that safinamide is a useful treatment option for PD patients suffering from motor fluctuations and some specific non-motor fluctuations. The broad beneficial effect of this compound can most likely be ascribed to its multimodal mechanism of action, which is dopaminergic (reversible MAO-B inhibition) and non-dopaminergic (modulation of the abnormal glutamate release). The efficacy of safinamide in early and advanced parkinsonian patients has been investigated in double blind, placebo controlled randomized clinical trials. In early PD patients16, safinamide 100 mg/d induced a significant improvement in the UPDRS part III score versus placebo (−6.0 points versus −3.6 (p = 0.0419). This positive trend was confirmed in the following double-blind, placebo-controlled extension study17. In contrast, when added to DAs at 200 mg a day, safinamide failed to provide a significant improvement in motor functions in early PD patients.
In advanced patients, safinamide was associated to a significant improvement of motor symptoms, evaluated through the UPDRS-III (motor score) administered during the ON period, in the three pivotal trials performed. In the 016 Study18, the UPDRS-III significantly improved, both in the safinamide 50 mg and 100 mg groups compared to placebo (LS mean changes for the 50 mg/day: −1.8 [95% CI, −3.3 to −0.4; p = 0.0138]; for the 100 mg/day: −2.6 in hours [95% CI, −4.1 to −1.1; p = 0.00060]). These results were confirmed and maintained throughout the double-blind, placebo-controlled, parallel group, extension study19. In the SETTLE trial20, when evaluated in the on phase, the mean UPDRS-III score was found to improve significantly in the safinamide 100 mg group compared to placebo: –3.43 (7.72) vs –1.83 (8.23), (LS mean difference, –1.82; 95% CI, –3.01 to –0.62; p = 0.003).
The improvement reported in the UPDRS was clearer in early patients but was less evident in fluctuators when UPDRS was administered during ON and was not the primary endpoint. In early PD patients, the improvement observed with safinamide at 200 mg daily failed to reach statistical significance, probably in relation to the higher discontinuations rate registered in the group treated at this dosage, which may have masked the clinical benefit.
Regarding the efficacy on motor fluctuations, a high level of evidence supports the consensus obtained in this Delphi panel on motor fluctuations. Safinamide has been demonstrated to significantly increase on time without dyskinesia and with non-troublesome dyskinesia compared with placebo, in both the 016 Study (least squares mean change versus placebo for safinamide 50 mg/day 0.51 h; 95% CI, 0.07–0.94; p = 0.0223 and for 100 mg/day 0.55 h; 95% CI, 0.12–0.99; p = 0.0130) and the 018 Study (LS increase from baseline: 1.01 h for the safinamide 50 mg/d group, 95% CI, 0.23, 1.11; p = 0.0031 and 1.18 h for the 100 mg/d group, 95% CI; 0.39, 1.27; p = 0.0002)18,19. A parallel, significant decrease in off time was also noted (−0.6, p = 0.0043 and −0.6, p = 0.0034) in the 016 trial. Moreover, a significant increase in on time without troublesome dyskinesia (+1.42, least-squares mean difference, 0.96 h; 95% CI, 0.56−1.37 h; p < 0.001) was reported in the SETTLE Study20. The efficacy of safinamide on motor fluctuations and motor symptoms was shown in an uncontrolled study on 91 patients, which reported a significant reduction in daily off time from baseline (median 60 min vs 30, p = 0.0001), and in on time with dyskinesia; a significant improvement from baseline was also found in UPDRS part III and UDysRS item 921. The recently published results of the SYNAPSES trial are in line with the literature, showing a progressive improvement of wearing off and early morning off over the 12 months study, a reduction in the percentage of fluctuating patients from 73.5% to 67.4%22 Two retrospective observational studies also highlighted the beneficial effect of this drug on fluctuations measured by the WOQ 1923 and CGI24.
The conflicting results obtained in this Delphi panel on dyskinesia reflect the uncertainty that emerged from clinical studies, where dyskinesia was the most frequently reported AE18,20. Indeed, in the 018 Study19 the primary endpoint, i.e., mean change from baseline (at Study 016 start18) to endpoint of the total score of the Dyskinesia Rating Scale (DRS) during on time, was not met. Nevertheless, a subsequent ad hoc analysis performed on moderately-to-severely dyskinetic patients who entered Study 016 (n = 242, 36%) showed a statistically significant reduction in the mean DRS total scores (p = 0.0317) and the same trend was observed in a post hoc analysis of the 018 study25, where safinamide, only when administered at 100 mg day, was found to improved DRS scores, regardless of LD changes. Moreover, in a retrospective study published recently, the introduction of safinamide was not associated with an improvement in the severity of dyskinesia, which remained unchanged in 85.4% of the enrolled population24. A potential anti-dyskinetic effect of safinamide may be related to a reduction in the excitatory overdrive of the direct pathway26. This characteristic distinguishes safinamide from amantadine, which is an anti-dyskinetic drug that directly inhibits glutamatergic receptors, but it is characterized by a somewhat unfavorable safety profile. Furthermore, a study to solely explore the effect of safinamide on dyskinesia has not been conducted yet and therefore it is difficult to infer a definite conclusion. The conflicting results on this topic may be also interpreted as an indication of the different therapeutic strategies adopted by neurologists when introducing safinamide in a therapeutic schedule, where the simultaneous adjustment of the concomitant dopaminergic drugs may have an important role in the severity of dyskinesia observed.
With respect to the glutamatergic system, all the panelists acknowledged that this plays an important role in PD aetiopathogenesis as well as in the emergence of its complications and that the anti-glutamatergic properties of safinamide may potentially explain an observed beneficial effect on dyskinesia and NMS. A dysfunction of both dopaminergic and non-dopaminergic pathways is known to contribute to development of NMS, thus drugs that interact with several neurotransmission systems may be helpful in alleviating these dysfunctions.
It has been suggested that the anti-glutamatergic properties of safinamide may be particularly beneficial in addressing motor complications and some specific NMS. This is a speculative comment but can be supported by animal models; there are data indicating the role of glutamatergic neurotransmission in modulating pain in normal and neuropathic rats27,28. Preclinical data suggest that the brain glutamate system may be involved in depression related behavior like anhedonia29. In humans with depression, neuroimaging studies found increased levels of glutamate in the putamen, highlighting a potential role of the basal ganglia in the neurophysiology of depression30. Glutamate was also hypothesized to influence the sleep-wake rhythm through the release of glutamate from the supra-mammillary region, which was associated to a sustained behavioral and EEG arousal response31.
A general consensus was reached on the efficacy of safinamide on pain, mood and sleep, based on literature findings. The possible effect of safinamide on NMS needs to be further explored in dedicated trials, since evidence currently available is limited32. Treatment with Safinamide was related to a long-lasting, significant reduction of pain-related sub-items of quality of life questionnaire (PDQ-39). Moreover, a reduction of concomitant pain killer treatment in fluctuating PD patients in a post-hoc analysis of the 018 study33 and in a pooled analysis of the 016 and SETTLE studies was observed34. A beneficial effect on pain was also shown in a small prospective study where safinamide was used to address pain related to motor fluctuations35. In a small retrospective analysis performed in fluctuating PD patients with sleep disruption, a significantly greater improvement of both, nocturnal sleep and diurnal sleepiness, was highlighted in the group of subjects treated with safinamide compared to those on rasagiline36.
Safinamide was found to significantly improve emotional well-being and depression for up to two years in a post hoc, pooled analysis of the 016 and 018 Studies37, even though in the original studies the improvement in the GRID Hamilton Rating Scale for Depression failed to reach statistical significance compared to placebo. In another retrospective study, safinamide was shown to significantly improve depressive symptoms in PD patient after just one month of treatment and to be well tolerated when co-administered with antidepressants38.
A beneficial effect on pain was shown in a retrospective survey and in a small prospective study35. In another retrospective study safinamide was shown to be useful for treating depressive symptoms in PD patients and safe when administered with concomitant antidepressant38. The positive effect on chronic pain and mood may be also related to the ability of safinamide to inhibit sodium channel39,40.
No agreement was obtained regarding domains like cognition, bladder, and sexual dysfunctions, highlighting the paucity of supporting evidence in these areas. Regarding cognition, in studies performed to explore the tolerability of safinamide in elderly and in general population, patients enrolled had no cognitive impairment (MMSE ≤ 23) or previous history of hallucinations;24 when patients with cognitive impairment were included, the number of withdrawals due to the occurrence of confusion was higher among patients with more severe cognitive impairment24. The lack of agreement on hallucinations reflects what emerged from post-marketing studies and in clinical practice where the use of safinamide was found to potentially increase the risk of hallucinations, most likely related to the higher dopaminergic load induced by the drug23,24.
The consensus on a likely lack of positive effects of safinamide on orthostatic hypotension was expected: even though the use of safinamide has been associated to a slight, non-significant raise in blood pressure values in pivotal trials18,19, the enhancements of the vasodilating action of dopaminergic drugs may explain the lack of tolerability observed in patients with OH. It has to be noted though that in a post-marketing study performed to evaluate the safety of the immediate switch from rasagiline to safinamide, blood pressure variability in systolic and diastolic values was unchanged between baseline and end of study 24-hour ambulatory blood pressure monitoring recordings41.
The beneficial effect of safinamide on QoL is supported by a high level of evidence. All studies that included QoL as an outcome parameter have demonstrated the efficacy of safinamide, when use at 100 mg, in benefiting both short-term and long-term quality-of-life outcomes in advanced PD patients measured by the PDQ-39 and the EQ5D questionnaires18,19,20,42,43.
The safety of safinamide was acknowledged by all panelists, in agreement with what had emerged from clinical trials in early16,17 and advanced PD patients18,19,20, where the majority of TEAE were mild to moderate in intensity and the most serious adverse events reported were judged as not related to the investigational drug. A large multinational, multicenter, retrospective, prospective 12-month observational cohort study evaluated the safety of safinamide in a real-life population of PD patients22 and showed a 30% lower rate of AEs compared to pivotal trials performed18,19,20. Subgroup analyses were performed for the following categories: age>75 years old, presence of relevant comorbidities and psychiatric conditions. Among these categories, SAEs were more frequent in patients aged >75 and AEs/SAEs were more frequent in those with relevant comorbidities. There were no cases of serotoninergic syndrome in participants treated with SSRI/SNRI or tricyclics, nor cases of impulse control disorders or sleep deterioration in patients taking concomitant dopamine-agonists. Dyskinesia was confirmed to be the most frequently reported AE, although observed at lower rates compared to the published RCTs (13.7% vs 18.3% for the 016 Study and 14.6% for the SETTLE)18,20. The SYNAPSES study22, conducted in a real word setting in six European countries, confirmed the safety and tolerability of safinamide, as adjunct therapy, in fluctuating patients and in special groups of subjects such as those suffering from neuropsychiatric symptoms (depression, bipolar disorders, psychosis and apathy).
The reversibility of the MAO-B inhibition of safinamide represents a clear advantage compared to the other MAO-B inhibitors when treating advanced PD patients with concomitant morbidities and high concomitant medications load, due to the reduced risk of drug interactions. Studies in healthy volunteers7,8 and patients44 have also confirmed that safinamide is not associated with any change of tyramine potentiation, with no risk of serotonin syndrome or hypertensive crisis.
Safinamide is a well-tolerated and easy to use drug thanks to its once-a-day administration. In fluctuating PD patients, this compound reduces OFF time without increasing troublesome dyskinesia. Its indirect anti-glutamatergic properties represent a novelty among the drugs for PD; its ability to reduce the hyperactivity of the direct pathway may potentially have long-term positive effect on the natural course of motor complications. Possibly due to its dual mechanism of action, it can be particularly useful in addressing NMS, whose treatment represents one of the current unmet needs of PD. Indeed, the ability to ameliorate NMS may be the most promising and distinctive feature of this compound: its potential efficacy in controlling pain, mood and sleep abnormalities may open the door to new therapeutics perspective if confirmed in randomized clinical trials.
The Delphi method requires participants to respond considering their personal experience/judgment on the proposed statements: the conclusions provided in this report are based therefore on the opinions of a panel of European movement disorder specialists, opinions maturated through a vast experience with the use of safinamide. The considerations highlighted in the paper may serve as a general reference for a more rational approach when prescribing safinamide, allowing movement disorders neurologists to further deepen, and not limit, their personal experience with this compound.
The consensus reached in this Delphi study provides an overview of how European movement disorders specialists view the role of safinamide in their clinical practice. The large agreement obtained reflects how specialists are mindful of the results of RCTs published and how these results guide their therapeutic perspective. The outcome almost perfectly reflects findings that have emerged in the literature and provides an interesting snapshot of the issues not yet addressed by the existing studies. Indeed, data on dyskinesia and NMS such as pain and mood are still limited, and more studies are needed to draw definite conclusion. However, clinical experience to date and the results of this Delphi process emphasizes that the data from controlled studies translate into a valuable clinical role of safinamide, which may be due to its dual mechanism of action, and this is true even at an early stage of motor fluctuations.
Methods
Study design and methods
The Delphi method is a survey technique that uses responses to a standardized questionnaire developed by a panel of experts to facilitate the convergence of opinions or the achievement of a common opinion in areas where scientific evidence is scarce or needed45,46,47. The Delphi method involves the repeated administration of questionnaires, where each statement can be evaluated through a five-point Likert scale, with a score from 1 to 5 (1, extremely disagree; 2, disagree; 3, agree; 4, mostly agree; and 5, extremely agree). Results are expressed as a percentage of respondents who scored each item as 1 or 2 (disagreement) or as 3, 4, or 5 (agreement). A positive consensus is reached if the percentage of agreement is greater than 66%. No consensus is reached, when the sum of the responses for a negative consensus (1 and 2) or a positive consensus (3, 4, and 5) is <66%.
Delphi process
Eight European countries were involved in the project which took place between October 2020 and January 2021. The survey was developed by a board of ten movement disorders specialists, who represent expert in the treatment of PD in their respective countries. After reviewing the published literature on safinamide, the Board met to discuss the main areas of interest and identified six major topics: the role of glutamate in D, introduction to fluctuations, efficacy of safinamide on motor symptoms, motor complications and NMS, Quality of Life, safety of safinamide and Target Population. Each topic was subdivided in a variable number of statements corresponding to items where greater need of clarification and debate existed. The Board was also asked to identify 2 colleagues for each board member who served as external impartial validators and judged clarity and readability of the statements. The survey was then distributed via an online platform to 119 panelists who met the following profile: movement disorders specialists with at least five years’ experience and using safinamide in their clinical practice. The vote was anonymous, and no compensation was given to any of the identified voters.
The study does not report on, or involve the use of any animal or human data or tissue, and does not contain data from any individual person, so there was no need for ethics approval nor prior approval of the study protocol. All experts involved in the Delphi survey were informed of the study’s objectives and the possibility of publishing the results in a peer-reviewed article. The participation was voluntary. They expressed their consent to participate in the survey after logging into the secure online survey platform via credentials, by actively clicking on the appropriate box.
Members of the validation panel
Valentina Leta (UK), Jasmin Rahimi (Austria), Katarina Rukavina (UK), Daniele Urso (UK)
Panelists
Araceli Alonso (Spain), Lucia Batzu (UK), Anna Rita Bentivoglio (Italy), Filip Bergquist (Sweden), Marta Blázquez Estrada (Spain), Alexandra Boogers (Belgium), Christof Brücke Brücke (Austria), Nuria Caballol Pons (Spain), Paolo Calabresi (Italy), Camille Carroll (Uk), Buhmann Carsten (Germany), Roberto Ceravolo (Italy), Roberto Cilia (Italy), Carlo Colosimo (Italy), Radu Constantinescu (Sweden), Pietro Cortelli (Italy), David Crosiers (Belgium), Beatriz De La Casa Fages (Spain), Gino De la Meilleure (Belgium), Mieke De Weweire (Belgium), Woitalla Dirk (Germany), Atbin Djamshidian (Austria), Laurens Dobbels (Belgium), Christian Dresel (Germany), Georg Ebersbach (Germany), Karla Eggert (Germany), Reinhard Ehret (Germany), Roberto Eleopra (Italy), Roberto Erro (Italy), Francisco Escamilla Sevilla (Spain), Giovanni Fabbrini (Italy), Thomas Foki (Austria), Pieret Françoise (Belgium), Rocío García-Ramos (Spain), Ransmayr Gerhard (Austria), Juan Carlos Gomez Esteban (Spain), Ayoze Nauzet González Hernández (Spain), Victoria Haunton (UK), Jorrit Hoff (Netherlands), Jagdish Sharma Jagdish (UK), Kassubek Jan (Germany), Maes Jen (Belgium), Leenders Jo (Belgium), Anders Johansson (Sweden), Winkler Juergen (Germany), Vinod Metta (UK), Ines Legarda (Spain), Johan Lökk (Sweden), Leonardo Lopiano (Italy), Michael Lorrain (Germany), Rosario Luquin (Spain), Martina Müngersdorf (Germany), Juan Carlos Martínez Castrillo (Spain), Pablo Mir (Spain), Thomas Müller (Germany), Nina De Klippel (Belgium), Bruggemann Norbert (Germany), Dag Nyholm (Sweden), Sven Pålhagen (Sweden), Santens Patrick (Belgium), Manuela Pilleri (Italy), Andrea Pilotto (Italy), Monika Pötter-Nerger (Germany), Rocco Quatrale (Italy), Silvia Ramat (Italy), Jason Raw (UK), Heinz Reichmann (Germany), Mario Giorgio Rizzone (Italy), Pilar Sanchez (Spain), Diego Santos García (Spain), Klaus Seppi (Austria), Ángel Sesar (Spain), Nishantha Silva (UK), Monty Silverdale (UK), Örjan Skogar (Sweden), Antonio Suppa (Italy), Per Svenningsson (Sweden), JP ter Bruggen (Netherlands), Alessandro Tessitore (Italy), Warnrecke Tobias (Germany), Lars Toenges (Germany), Volker Tomantschger (Austria), Thomas Vaterrodt (Germany), Pieter Viaene (Belgium), Dieter Volc (Austria), Hofmann W. E. (Germany), Karoline Wenzel (Austria), Christian Winkler (Germany), Mario Zappia (Italy), Anna Lena Zecchinelli (Italy).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fox, S. H. Movement Disorder Society Evidence-Based Medicine Committee et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 33, 1248–1266 (2018).
Alborghetti, M. & Nicoletti, F. Different generations of Type-B monoamine oxidase inhibitors in Parkinson’s Disease: from bench to bedside. Curr. Neuropharmacol. 17, 861–873 (2019).
Moussa, B. H., Bakhle, Y. & Bakhle, Y. S. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 147, S287–S296 (2006).
Marzo, A. et al. Pharmacokinetics and pharmacodynamics of safinamide, a neuroprotectant with antiparkinsonian and anticonvulsant activity. Pharmacol. Res. 50, 77–85 (2004).
Chazot, P. L. Safinamide (Newron Pharmaceuticals). Curr. Opin. Investig. Drugs 2, 809–813 (2001).
Caccia, C. et al. Safinamide: from molecular targets to a new anti-Parkinson drug. Neurology 67, S18–S23 (2006).
Cattaneo, C. et al. Pressor response to intravenous tyramine in healthy subjects after safinamide, a novel neuroprotectant with selective, reversible monoamine oxidase B inhibition. Clin. Neuro Pharmacol. 26, 213 (2003).
Marquet, A. et al. The effect of safinamide, a novel drug for Parkinson’s disease, on pressor response to oral tyramine: a randomized, double-blind, clinical trial. Clin. Pharmacol. Ther. 92, 450–457 (2012).
Giossi, R. et al. Overall efficacy and safety of safinamide in Parkinson’s Disease: a systematic review and a meta-analysis. Clin Drug Investig. 41, 321–339 (2021).
Stocchi, F. & Torti, M. Adjuvant therapies for Parkinson’s disease: critical evaluation of safinamide. Drug Des. Devel Ther. 10, 609–618 (2016).
Kandadai, R. M., Jabeen, S. A., Kanikannan, M. A. & Borgohain, R. Safinamide for the treatment of Parkinson’s disease. Expert Rev. Clin. Pharmacol. 7, 747–759 (2014).
Jenner, P. & Caccia, C. The role of glutamate in the healthy brain and in the pathophysiology of Parkinson’s Disease. European Neurological Review 14(Suppl.2), 2–12 (2019).
Gonzalez-Latapi, P., Bhowmick, S. S., Saranza, G. & Fox, S. H. Non-dopaminergic treatments for motor control in parkinson’s disease: an update. CNS Drugs 34(Oct), 1025–1044 (2020).
Stocchi, F. DEEP study group et al. Early detection of wearing off in Parkinson disease: the DEEP study. Parkinsonism Relat. Disord. 20, 204–211 (2014).
Stacy, M. et al. End-of-dose wearing off in Parkinson disease: a 9-question survey assessment. Clin. Neuropharmacol. 29, 312–321 (2006).
Stocchi, F. et al. Study 015 Investigators. A randomized, double-blind, placebo controlled trial of safinamide as add on therapy in early Parkinson’s disease patients. Mov. Disord. 27, 106–112 (2012).
Schapira, A. H. V. et al. Study 017 Investigators. Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson’ disease. Eur. J. Neurol. 20, 271–280 (2013).
Borgohain, R. et al. Study 016 Investigators. Randomized trial of safinamide add-on to Levodopa in Parkinson’s disease with motor fluctuations. Mov. Disord. 29, 229–237 (2014).
Borgohain, R. et al. Study 018 Investigators. Two year, randomized, controlled study of Safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov. Disord. 29, 1273–1280 (2014).
Schapira, A. H. et al. Assessment of Safety and Efficacy of Safinamide as a Levodopa Adjunct in Patients With Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol. 74, 216–224 (2017).
Mancini, F. et al. Real life evaluation of safinamide effectiveness in Parkinson’s disease. Neurol. Sci. 39(Apr), 733–739 (2018).
Abbruzzese, G. et al. SYNAPSES Study Investigators Group. A European observational study to evaluate the safety and the effectiveness of safinamide in routine clinical practice: the SYNAPSES Trial. J Parkinsons Dis 11, 187–198 (2021).
Rinaldi, D. et al. The tolerability, safety and efficacy of safinamide in elderly Parkinson’s disease patients: a retrospective study. Aging Clin Exp Res. 33, 1689–1692 (2021).
Martí-Andrés, G. et al. Safinamide in clinical practice: a Spanish multicenter cohort study. Brain Sci. 9(Oct), 272 (2019).
Cattaneo, C. et al. Long-term effects of safinamide on dyskinesia in mid-to late-stage Parkinson’s disease: a post-hoc analysis. J. Parkinsons Dis. 5, 475–481 (2015).
Blandini, F., Porter, R. H. & Greenamyre, J. T. Glutamate and Parkinson’s disease. Mol. Neurobiol. 12, 73–94 (1996).
Chen, S. R. & Pan, H. L. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured rats. J. Pharm. Exp. Ther. 312, 120–126 (2005).
Marabese, I. et al. Metabotropic glutamate receptor subtype 7 in the dorsal striatum oppositely modulates pain in sham and neuropathic rats. Neuropharmacology 135, 86–99 (2018).
Mao, L. M. & Wang, J. Q. Alterations in mGlu5 receptor expression and function in the striatum in a rat depression model. J. Neurochem. 145, 287–298 (2018).
Godlewska, B. R. et al. Brain glutamate in medication-free depressed patients: a proton MRS study at 7Tesla. Psychol. Med. 48, 1731–1737 (2018).
Pedersen, N. P. et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat. Commun. 8, 1405 (2017).
Seppi, K. et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. 26(Oct), S42–S80 (2011). Suppl 3(0 3).
Cattaneo, C., Kulisevsky, J., Tubazio, V. & Castellani, P. Long-term efficacy of safinamide on parkinson’s disease chronic pain. Adv. Ther. 35, 515–522 (2018).
Cattaneo, C., Barone, P., Bonizzoni, E. & Sardina, M. Effects of safinamide on pain in fluctuating parkinson’s disease patients: a post-hoc analysis. J. Parkinsons Dis. 7, 95–101 (2017).
Geroin, C. et al. Effects of safinamide on pain in Parkinson’s disease with motor fluctuations: an exploratory study. J. Neural Transm. (Vienna). 127, 1143–1152 (2020).
Liguori, C., Stefani, A., Runi, R., Mercuri, N. B. & Pierantozzi, M. Safinamide effect on sleep disturbances and daytime sleepiness in motor fluctuating Parkinson’s disease patients: A validated questionnaires-controlled study. Park. Relat. Disord. 57, 80–81 (2018).
Cattaneo, C. et al. Long-term effects of safinamide on mood fluctuations in Parkinson’s Disease. J. Parkinsons Dis. 7, 629–634 (2017).
Pena, E. et al. Impact of safinamide on depressive symptoms in Parkinson’s Disease Patients (SAD-ness PD Study]: a multicenter retrospective study. Brain Sciences 11, 232 (2021).
Bourin, M., Chenu, F. & Hascoët, M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr. Drug Targets 10(Nov), 1052–1060 (2009).
Bhattacharya, A., Wickenden, A. D. & Chaplan, S. R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 6(Oct), 663–678 (2009).
Stocchi, F. et al. Overnight switch from rasagiline to safinamide in Parkinson’s disease patients with motor fluctuations: a tolerability and safety study. Eur. J. Neurol. 28, 349–354 (2021).
Cattaneo, C., Jost, W. H. & Bonizzoni, E. Long-term efficacy of safinamide on symptoms severity and quality of life in fluctuating parkinson’s disease patients. J. Parkinsons Dis. 10, 89–97 (2020).
Martinez-Martin, P., Rodriguez-Blazquez, C., Forjaz, M. J. & Kurtis, M. M. Impact of pharmacotherapy on quality of life in patients with Parkinson’s Disease. CNS Drugs May 29, 397–413 (2015).
Stocchi, F. et al. A randomized, double-blind, placebo-controlled trial of safinamide as add on therapy in early Parkinson’s disease patients. Mov. Disord. 27, 106–112 (2012).
Fowles, J. Handbook of futures research. (Greenwood Press, 1978).
Giannarou, L. & Zervas, E. Using Delphi technique to build consensus in practice. Int J. Bus. Sci. Appl Manag. 9, 65–82 (2014).
Walker, A. & Selfe, J. The Delphi method: a useful tool for the allied health researcher. Br. J. Ther. Rehabilitation. 3, 677–681 (1996).
Acknowledgements
The authors wish to thank Ethos srl for logistic support in conducting the Delphi study. This publication was supported by an unrestricted grant from Zambon.
Author information
Authors and Affiliations
Contributions
F.S., A.A., D.B., B.B., W.J., R.K., J.K., P.O., F.V. and K.R.C. drafted the manuscript. F.S., A.A., D.B., B.B., W.J., R.K., J.K., P.O., F.V. and K.R.C. contributed to formulating the questionnaire, analyzing the responses, and made critical revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Fabrizio Stocchi has received compensation for consultancy and speaker related activities from Zambon, UCB, Chiesi Pharma, Lundbeck, Sunovion, Bial, SynAgile, Biogen, Kiowa and Impax. Angelo Antonini has received compensation for consultancy and speaker related activities from UCB, Boehringer Ingelheim, General Electric, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Neuroderm, Theravance Biopharma, Roche, Medscape; he receives research support from Bial, Lundbeck, Roche, Angelini Pharmaceuticals, Horizon 2020 - Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer–Ingelheim for legal cases on pathological gambling. Daniela Berg reports grants from Janssen Pharmaceutica, grants from the Damp foundation, grants from the German Parkinson’s Disease Association (dPV), grants from BMWi, grants from BMBF, grants from the ParkinsonFonds Deutschland GmbH, grants and speaker’s honoraria from and consultancy honoraria of UCB Pharma GmbH, grants from and consultancies for Novartis Pharma GmbH, grants and speaker’s honoraria from and consultancy honoraria of Lundbeck, speaker’s honoraria from and consultancy honoraria of BIAL, speaker’s honoraria from and consultancy honoraria of Biogen, honoraria from Bayer and Zambon outside the submitted work. Bruno Bergmans has received grants and compensation for lectures and advisory functions from Abbvie, Zambon, Merz Pharma, Orion, Allergan and St Jude. Wolfgang Jost is advisor and speaker for Abbvie. Bial, Desitin, Stada, UCB, Zambon. Regina Katzenschlager has received honoraria for speaking and consulting from AbbVie, AOP Orphan, Bial, Britannia, Ever Pharma, Stada, UCB, and Zambon, and research grants from Britannia, Stada and Zambon within the past 3 years. Pier Odin has received compensation for lectures and advisory functions from AbbVie, Bial, Britannia, Ever Pharma, Lobsor, Nordic Infucare, Stada and Zambon. Jaime Kulisevsky: No conflicts regarding this manuscript. K. Ray Chaudhuri: Advisory board: AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion, Roche, Therevance, Scion, Britannia, Acadia, 4D. Honoraria for lectures: AbbVie, Britannia, UCB, Zambon, Novartis, Boeringer Ingelheim, Bial; Grants (Investigator Initiated): Britania Pharmaceuticals, AbbVie, UCB, GKC, Bial, Academic grants: EU, IMI EU, Horizon 2020, Parkinson' UK, NIHR, PDNMG, EU (Horizon 2020), Kirby Laing Foundation, NPF, MRC, Wellcome Trust.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stocchi, F., Antonini, A., Berg, D. et al. Safinamide in the treatment pathway of Parkinson’s Disease: a European Delphi Consensus. npj Parkinsons Dis. 8, 17 (2022). https://doi.org/10.1038/s41531-022-00277-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00277-z
This article is cited by
-
Safinamide, an inhibitor of monoamine oxidase, modulates the magnitude, gating, and hysteresis of sodium ion current
BMC Pharmacology and Toxicology (2024)
-
Parkinson’s disease therapy: what lies ahead?
Journal of Neural Transmission (2023)
-
Fatigue in fluctuating Parkinson’s disease patients: possible impact of safinamide
Journal of Neural Transmission (2023)
-
Why do ‘OFF’ periods still occur during continuous drug delivery in Parkinson’s disease?
Translational Neurodegeneration (2022)