Abstract
In this study, SiO2 coating was electrodeposited on Ti45Al8.5Nb alloy to enhance its oxidation resistance at 900 °C. The focus was on the interaction between SiO2 coating and the alloy substrate, and specifically, the role of the Nb element in this context. The formation of a SiO2/(Ti, Nb)O2/Ti5Si3 + Al2O3 three-layer oxide scale significantly inhibits the inward diffusion of oxygen. Beyond the characteristic Ti5Si3 + Al2O3 layer at the interface, the Nb2Al phase embedded within Ti5Si3 was observed. Concurrently, the presence of a (Ti, Nb)O2 layer was confirmed. The relationship between the coating thickness and oxidation resistance was also investigated. It was found that the thickness of the SiO2 coating affects the density of the oxide scale and the inward diffusion rate of oxygen. Moreover, due to the good adhesion to the substrate, the derived oxide scale exhibited good anti-peeling performance when subjected to a cyclic oxidation test.
Similar content being viewed by others
Introduction
Titanium aluminide alloys have garnered attention as potential substitutes for nickel-based alloys in the aerospace industry due to their low density, high specific strength, and excellent resistance to high-temperature creep1,2,3,4,5. However, the loose and unprotected mixed oxide layer consisting of TiO2 and Al2O3 will be generated when TiAl alloy is serviced at high temperatures and aerobic environments6,7, therefore hindering their industrial application8.
Alloying with Nb has been shown to significantly enhance the oxidation resistance of TiAl alloys. The specific role of Nb includes enhancing the activity of Al to facilitate the formation of Al2O3 protective scales, substituting Ti atoms in TiO2 to reduce oxygen vacancy concentration, and promoting the formation of TiN to inhibit outward diffusion of Ti9,10. Nevertheless, the initial Al2O3 scales formed on high Nb-containing TiAl alloys exhibit inadequate adhesion to the substrate during prolonged service, failing to provide sufficient protection. At elevated temperatures, severe oxidation and scale spalling occur. Researchers have introduced alloying elements such as B11,12, Y13, Ta14, and C15 into high Nb-containing TiAl alloys to address this issue. These elements benefit to refine the grain structure or pin grain boundaries, enhancing the adhesion of oxide scales. For instance, Xiang et al. found that moderate additions of Y to high Nb-containing TiAl alloys resulted in Y segregation at grain boundaries or within grains. Subsequent oxidation to form Y2O3 contributed to grain refinement and pinning, improving the resistance to scale spalling13. Our previous studies have shown that nano-SiO2 particles can react with high Nb-containing TiAl alloy and enhance oxidation resistance16.
However, excessive alloying elements may degrade the mechanical properties of the alloy13,17. More direct approaches involve surface modification18,19,20,21,22 and application of protective coatings23,24,25,26,27,28,29. For example, Crespo-Villegas et al. reported that the magnetron sputtered TixSiy coating inhibited the expansion of the TiO2 oxide scale and reduced the oxidation rate of TiAl alloy23. The TiAlCrY coating prepared by Han et al. exhibited good chemical compatibility with the TiAl substrate, therefore improving the oxidation resistance of the TiAl alloy30. Yang et al. found the arc ion-plated AlN coating could be oxidized to protective Al2O3 scale, which has good adhesion to TiAl alloy due to the generation of Ti3Al/Ti2AlN/Ti3Al composite layer between the Al2O3 scale/TiAl alloy interface24.
Owing to the low diffusion rate for oxygen and good thermal stability, SiO2-based ceramic coating shows good oxidation performance for Ti31,32 and TiAl alloys33. In 1993, SiO2 coating with 0.2 μm was prepared by dip-coating to improve the oxidation resistance of TiAl alloy in pure oxygen33. Results showed that the SiO2 coating could dramatically reduce the oxidation rate of TiAl alloy at 1100 K and 1200 K due to the formation of an Al2O3 layer beneath the SiO2 coating. Whereas, the dip-coated and spin-coated SiO2 coatings have limited thickness and are prone to peel off from the substrate due to poor adhesion33,34,35. Recently, we demonstrated that based on the chemical bonding feature, the cathodic electrodeposited SiO2 coating exhibited good adhesion properties to the substrate and dramatically improved the oxidation resistance of Ti50Al alloy36,37. Our previous research revealed that at 900 °C, evident mutual diffusion and chemical reactions are occurring between the electrodeposited SiO2 coating and the TiAl alloy, resulting in the formation of a multilayered composite structure at the interface36. This structure enhances the adhesion of SiO2 coating to the substrate and buffers the difference in thermal expansion coefficients between the coating and the substrate. Additionally, the introduction of K into the electrolyte during the preparation of electrodeposited SiO2 coating promotes viscous flow at high temperatures, reducing its brittleness. However, the synergistic effects between SiO2 and Nb require further investigation.
The present work aims to evaluate the oxidation performance of electrodeposited SiO2 coating on Ti45Al8.5Nb alloy. The interaction between the SiO2 coating and Ti45Al8.5Nb alloy was investigated. An in-depth exploration of the influence of coating thickness on the interaction between the coating and the substrate was evaluated. Results show that Nb is dispersed within Ti5Si3 as Nb2Al crystal grains, curtailing the formation of the Z phase. At the same time, the diffusion of Nb into the coating leads to the formation of a (Ti, Nb)O2 layer in the Ti-rich layer, enhancing the compactness of the TiO2 layer structure. The synergistic action of SiO2 and Nb enhances the oxidation resistance of the alloy.
Results
Structure and morphology of the SiO2 coating
SiO2 coatings composed of spherical particles have a porous structure (Supplementary Fig. 2), which is caused by hydrogen evolution reaction during the electrodeposition process38. It is revealed that the size of the spherical particles increases with the current density due to the enhanced sol-gel condensation process (Supplementary Fig. 2a, c, and e). Notably, the stress concentration in the coating with high thickness brings about a tendency to crack. A similar phenomenon can be found when extending the electrodeposition time (data not shown).
The cross-sectional BSE images of SiO2 coatings prepared under different current densities are characterized (Supplementary Fig. 3). The thickness of the coatings increases in correlation with the current density, as indicated. Specifically, the thickness of the SiO2 coatings increases from approximately 3.8 μm to 10.8 μm as the current density rises from 0.5 mA cm−2 to 3 mA cm−2.
Before oxidation at 900 °C, the SiO2 coating was sintered at 800 °C for 5 h. The top surface scanning electron microscope (SEM) image (Fig. 1a) and cross-sectional BSE image (Fig. 1b) reveal that the porous SiO2 coating (Supplementary Fig. 2d) turns out to be dense after the sintering treatment. Simultaneously, cracks can be found on the surface, which is attributed to the release of thermal stress during the sintering process (Fig. 1a). Notably, these deformations do not pervade the full depth of the coating. Under elevated temperatures, a gradual self-healing process may be anticipated36. Moreover, the coating thickness diminishes from 4.5 μm to 3.2 μm due to the shrinking effect (Fig. 1b). As demonstrated in Fig. 1c through the EDS elements line scan and in Fig. 1d by the elements distribution mapping, the SiO2 coating and the substrate alloy are well combined, and there is a clear interface between them. The element K is derived from the electrolyte. It should be noted that although we can see from Fig. 1d that there seems to be a K element in the matrix, this is actually caused by the EDS measurement deviation. The matrix used in this experiment did not incorporate the K element.
Oxidation behavior of SiO2 coating at 900 °C under isothermal conditions
The kinetic curves of the SiO2 coatings prepared under different parameters are presented in Fig. 2. The curves indicate that the SiO2 coating prepared with suitable parameters can provide good oxidation resistance for Ti45Al8.5Nb alloy. After oxidizing for 100 h, the uncoated Ti45Al8.5Nb alloy experiences a weight gain of 0.7 mg cm−2 (curve 1). For the SiO2 coating electrodeposited for 300 s, the weight gain of the coating protective alloy decreases first but then increases as the current density increases from 0.5 to 3 mA cm−2 (Fig. 2a).
The mass gain of bare Ti45Al8.5Nb alloy and SiO2 coating prepared at 0.5 mA cm−2 can be separated into two stages. Specifically, the weight gain increases dramatically in the initial 10 h oxidation stage, and then the oxidation rate slows down. Because a protective Al2O3 layer is generated on the Ti45Al8.5Nb alloy during the early stage of oxidation9,10. For the SiO2 coating prepared at low current density, only limited improvement could be achieved because the derived coating is relatively thin. When the current density is further elevated to 1 mA cm−2, the minimum mass gain of 0.42 mg cm−2 is achieved (curve 3). Further increasing the current density leads to the deterioration of the oxidation resistance (curve 4). As mentioned above, cracks are generated on the coating when the current density is high (Supplementary Fig. 2). These cracks serve as diffusion pathways for oxygen and nitrogen to reach the substrate. Similarly, the mass gain of the coating-alloy system initially decreases but then increases with the electrodeposition time (Fig. 2b). This phenomenon will be further explained in the subsequent analysis. The specimen electrodeposited at 1 mA cm−2 for 300 s exhibits the best oxidation performance.
The oxidation performance of various coatings fabricated on high Nb-containing TiAl alloys was summarised in Supplementary Table 1. It is revealed that the SiO2 coating exhibits a superior oxidation resistance (0.42 mg cm−2) to NiCoCrAlYMo coating (1.99 mg cm−2)39, CoNiCrAlTaY coating (0.78 mg cm−2)40, Al2O3 coating (0.5 mg cm−2)41,42, Al2O3–TiO2–NiCoCrAlY coating (0.88 mg cm−2)43, and YSZ–TiAlCr coating (>0.6 mg cm−2)44. However, certain coatings, including Al2O3–Y2O3 coating (0.2 mg cm−2)45, Ni-doped Al2O3 coating (0.2 mg cm−2)46, and Ni coating (0.34 mg cm−2 when oxidized for 90 h)47 showcase exceptional oxidation resistance.
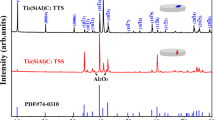
The X-ray diffraction (XRD) patterns of the bare alloy and SiO2 coatings subjected to isothermal oxidation under various electrodeposition conditions are presented in Fig. 3. After 100 h oxidation, the diffraction peaks corresponding to TiAl, TiN, TiO2, and Al2O3 are observed in the bare Ti45Al8.5Nb alloy. For SiO2 coatings, extra diffraction peaks assigned to Ti5Si3 and two polymorphs of SiO2 are detected. Ti5Si3 arises from the reactions of Ti and Al with SiO248,49.
The formation mechanism will be further elaborated in the discussion section. The coatings produced under different electrodeposition parameters exhibit comparable diffraction profiles. Interestingly, the intensity of TiO2 diffraction within the SiO2 coating is notably lower compared to that in the uncoated Ti45Al8.5Nb alloy, suggesting that the SiO2 coating restrains the expansion of TiO2.
The top surface SEM images of the specimens after undergoing 100 h of isothermal oxidation are exhibited in Fig. 4. In conjunction with EDS analysis (area 1 in Fig. 4a and Table 1) and XRD results (Fig. 3), bare Ti45Al8.5Nb is covered by equiaxed crystals comprising of TiO2 and Al2O3 (Fig. 4a, b). According to EDS analyses (area 2 in Fig. 4c and Table 1) and the XRD pattern (Fig. 3), the SiO2 coating of 0.5 mA cm−2 is primarily covered with Al2O3, TiO2, and SiO2. Needle-like oxide scale is found on the top surface (Fig. 4c), which is dramatically different from the bare alloy (Fig. 4a). Concerning the SiO2 coating created with a current density of 1 mA cm−2, in addition to the dense SiO2 coating area (point 1 in Table 1 and Fig. 4f)), a mixture of TiO2 and Al2O3 particles appeared (point 2 in Table 1 and Fig. 4f) emerge. This indicates that Ti and Al diffuse through the SiO2 coating and are oxidized at the surface. However, the SiO2 coating remains intact (Fig. 4e, f). For the SiO2 coating deposited at 3 mA cm−2, the oxide scale is primarily composed of SiO2 (points 3 and 4 in Table 1 and Fig. 4h). Nonetheless, cracks are observed (Fig. 4h), possibly caused by the mismatch in expansion coefficients of the coating and substrate. These cracks would diminish the protective performance of the SiO2 coating.
In Fig. 5a, the cross-sectional BSE image of the bare Ti45Al8.5Nb alloy displays an oxide layer with a thickness of 5 µm. EDS analysis (point 1 in Table 2), line scanning (Fig. 5b), and mapping distribution (Fig. 5c) reveal that the outermost layer is Al enriched, aligning with the Al2O3 phase identified via XRD (Fig. 3). It has been reported that Nb intensifies the activity of Al, leading to the preferential oxidation in the initial stage of oxidation50. Then a Ti and Nb oxides mixture layer is found, which is supported by the EDS result (point 2 in Table 2). The innermost layer, enriched with Ti and identified as TiN via XRD, is generated as a result of the inward diffused N2 to the oxide-matrix interface51. The EDS result also shows this layer is Ti and N enriched (point 3 in Table 2). Although an Al2O3 outer layer is generated, limited protectiveness is achieved due to its discontinuous structure.
The cross-sectional BSE image and EDS analyses of the specimen fabricated at 0.5 mA cm−2 for 300 s and subsequently oxidized at 900 °C for 100 h were characterized (Supplementary Fig. 4). Combined with the XRD pattern (Fig. 3) and EDS results (Supplementary Fig. 4), it can be concluded that the oxide scale resembles that of the bare alloy and consists of Al2O3 (point 1 in Supplementary Table 2), TiO2 (point 3 in Supplementary Table 2), and TiN (point 4 in Supplementary Table 2) from the surface inward. However, a noteworthy difference is the presence of Si distribution in the outermost Al2O3 (point 1 in Supplementary Table 2). EDS point analysis shows the existence of SiO2 in the oxide scale (point 2 in Supplementary Table 2). Additionally, the TiO2 layer is noticeably thinner, implying that the SiO2 coating plays a role in limiting TiO2 growth. EDS line scanning (Supplementary Fig. 4b), and mapping distribution (Supplementary Fig. 4c) further demonstrate that the formation of a thicker Al2O3 layer is promoted by the SiO2 coating. Despite this, significant interdiffusion within the oxide layer suggests the failure of SiO2 coating to impede oxidation effectively.
The cross-sectional BSE image and EDS assessments of the specimen fabricated at 1 mA cm−2 for 300 s after oxidation are presented in Fig. 6. When contrasted with the cross-sectional BSE image after sintering (Fig. 1b), it can be found that there is no considerable alteration in coating thickness, retaining an estimated value of about 3.3 μm. The SiO2 coating is compact and continuous (Fig. 6a and point 1 in Table 3). The noteworthy deviation lies in the emergence of a thin interdiffusion zone at the interface of coating and alloy, as indicated by EDS line scanning (Fig. 6b). This layer includes diffusion of Si from the coating to the matrix and Ti, Al, and Nb from the substrate to the SiO2 coating (point 2 in Table 3), thus enhancing the adhesion properties of the SiO2. Additionally, at the boundary between the diffusion zone and the substrate (Fig. 6c), a TiN layer is discerned (point 3 in Table 3), which is consistent with the XRD result (Fig. 3). Collectively, the SiO2 coating maintains its compactness and integrity after oxidation for 100 h, effectively hindering the inward diffusion of oxygen to the substrate.
The cross-sectional BSE image of the specimen prepared at 3 mA cm−2 for 300 s (Fig. 7) shows significant differences after oxidation. Beneath the SiO2 coating (point 1 in Table 4), there is a subsequent layer consisting of TiN followed by an internal oxidation zone with a thickness of approximately 12.5 μm (Fig. 7a, b). EDS result also demonstrates the existence of elements Ti and N (point 2 in Table 4). Combining the XRD pattern (Fig. 3) with the elements distribution mapping (Fig. 7c), banded TiO2 and Al2O3 appear to stagger within this region, while Nb segregates towards the banded TiO2. The occurrence of internal oxidation can be attributed to the generation of numerous cracks during the SiO2 coating preparation, which provides pathways for early oxygen diffusion to the interior. Moreover, traces of Ti are observed in the SiO2 coating, indicating the growth of TiO2 within the cracks during the early stages.
After oxidizing for 100 h at 900 °C, TEM was used to obtain detailed structure and composition information of the oxide scale in specimens fabricated at 1 mA cm−2 for 300 s (Fig. 8). The oxide layer can be divided into three distinct regions based on the contrasting features in the high-angle annular dark-field (HAADF) image (Fig. 8a) and the elements distribution mapping (Fig. 8b). The outermost layer consists of amorphous silica interspersed with crystalline particles. This is supported by multiple observations. Firstly, diffraction peaks corresponding to tridymite and cristobalite are identified in the XRD pattern (Fig. 3). Secondly, in the selected area electron diffraction (SAED) image, bright spots are observed (Fig. 8a1). These bright spots can be attributed to specific interplanar spacings measured after the Fast Fourier Transform (FFT), which are 0.403 nm (associated with the cristobalite (101) plane) and 0.249 nm (associated with the cristobalite (200) plane).
The HAADF image (a), and the elements distribution mapping (b) of the SiO2 coating after isothermal oxidation at 900 °C for 100 h. The SiO2 coating was electrodeposited at 1 mA cm−2 for 300 s. The high-resolution TEM images and SAED of the positions marked in (a) are displayed as a1–a6, respectively.
Beneath this layer, a layer consisting of rutile-TiO2 is identified by a high-resolution TEM image and SAED pattern (Fig. 8a3). The interplanar spacings recorded at 0.254 nm and 0.205 nm align with the (101) and (210) crystal planes of rutile-TiO2, respectively. Beneath the TiO2 layer, there is a layer existing alternating light and dark regions. The bright region, rich in Ti, Si, and Nb as indicated by elements distribution mapping (Fig. 8b), is identified as Ti5Si3 and Nb2Al through high-resolution imaging and SAED pattern analysis (Fig. 8a4). The interplanar spacings observed at 0.220 nm and 0.245 nm are associated with the Ti5Si3 (211) and Ti5Si3 (210) planes, respectively. Meanwhile, the 0.233 nm spacing is attributed to the Nb2Al (330) plane. Similarly, high-resolution TEM image and SAED pattern analysis confirm the dark region to be α-Al2O3, with the detected interplanar spacings of 0.235 nm and 0.216 nm being consistent with the α-Al2O3 (110) and α-Al2O3 (006) planes, respectively (Fig. 8a5). In addition, the polycrystalline diffraction rings in the SAED diagram correspond to the two interplanar spacings of α-Al2O3 (104) and α-Al2O3 (012), respectively.
Apart from the above three layers, a discontinuous Al-based layer can be found between the SiO2 layer and the TiO2 layer, labeled as a2 (Fig. 8a). This region is identified as AlNbO4, as supported by the matched interplanar spacing of AlNbO4 (104) in the high-resolution images (Fig. 8a2). The SAED pattern exhibits diffraction rings corresponding to the AlNbO4 (11–1) and AlNbO4 (11–2) planes, respectively. Furthermore, the interplanar spacing of 0.170 nm observed in region 6 corresponds to the TiAl alloy (Fig. 8a6).
As mentioned earlier, after oxidized at 900 °C for 100 h, there is an internal oxidation zone for the specimen prepared at 3 mA cm−2 for 300 s (Fig. 7). TEM was performed in this region to determine its compositional distribution (Fig. 9). The focused ion beam (FIB) cutting position and morphology of the final thinned specimen are shown in Fig. 9a, b, respectively. The HAADF image displays numerous Kirkendall voids within this region (Fig. 9c). By comparing the interplanar spacing in the area marked as c1, it is confirmed to be Nb2Al (Fig. 9c1). The measured interplanar spacing of 0.336 nm corresponds to the Nb2Al (211) plane, and the diffraction spots in the SAED correspond to the Nb2Al (211) and Nb2Al (101) planes, respectively.
The SiO2 coating/alloy interface region (a), the final thinned sample prepared by FIB (b), the HAADF image (c), the high-resolution TEM images and SAEDs of the marked positions (c1 and c2), and the elements distribution mapping of c2 (d). The SiO2 coating was electrodeposited at 3 mA cm−2 for 300 s.
A light and dark mixed region was selected for elemental analysis by EDS, as shown in Fig. 9d. The dark area shows a concentration of Al, while the bright region displays an enrichment of Ti and Nb. SAED and high-resolution images were obtained for the bright and dark regions, respectively. As seen in Fig. 9c2, the interplanar spacing of 0.352 nm corresponds to the α-Al2O3 (012) plane, whereas the rutile-TiO2 (101) plane is associated with a spacing of 0.257 nm. These indicate that the dark region corresponds to Al2O3, while the bright region corresponds to TiO2. This finding explains why the specimens prepared at 3 mA cm−2 do not have complete coverage of either Al2O3 or TiO2 on the surface, yet exhibit a higher mass gain compared to the bare alloy. The insufficiently dense structure of the coating permits oxygen ingress to the boundary between the coating and substrate, leading to substrate oxidation at elevated temperatures. This internal oxidation hinders the development of a protective Al2O3 layer over the substrate as it would be in the case of the bare specimen. Moreover, the segregation of Al, Ti, and Nb results in the creation of numerous voids within the internal oxidation zone, further exacerbating the oxidation process.
Cyclic oxidation behavior of SiO2 coating at 900 °C
Figure 10 presents the mass gain curves and macroscopic morphology of the SiO2 coating fabricated at 1 mA cm−2 for 300 s after cyclic oxidation at 900 °C for 200 h, as well as the bare alloy. The mass gain curves and optical images indicate that the oxide layer of the bare alloy starts to peel off after 140 h of cyclic oxidation (curves 1 and 2), while the SiO2 coating remains intact even after 200 h of cyclic oxidation (curve 3). Additionally, it can be observed that the mass gain of the SiO2 coating exhibits a slowdown after 100 cycles, suggesting the formation of a stable protective film on its surface.
After the cyclic oxidation test, TiAl, TiO2, Al2O3, and Nb2Al are identified from both bare Ti45Al8.5Nb (Supplementary Fig. 5a) and SiO2-coated specimens (Supplementary Fig. 5b). Whereas, TiO2 and Al2O3 exhibit more pronounced diffraction peaks on bare Ti45Al8.5Nb alloy, suggesting the bare alloy undergoes more intense oxidation. Notably, two extra crystal forms of SiO2 are identified from the SiO2-coated specimen.
The surface morphology of the bare Ti45Al8.5Nb alloy and SiO2 coating after cyclic oxidation for 200 h at 900 °C is investigated (Supplementary Fig. 6). For bare Ti45Al8.5Nb alloy, severe spallation is found at the local region (Supplementary Fig. 6a). The unpeeled region shown in Supplementary Fig. 6a1 is covered with equiaxed oxide mainly composed of TiO2, as confirmed by XRD pattern (Supplementary Fig. 5a) and EDS analysis (point 1 in Supplementary Table 3). In contrast, the oxide scale on the spallation region shown in Supplementary Fig. 6a2 appears flocculent and consists primarily of Al2O3 and TiO2, as determined by XRD pattern (Supplementary Fig. 5b) and EDS analysis (points 2 and 3 in Supplementary Table 3). In contrast, the oxide scale on the surface of the SiO2 coating is continuous and consists of uniform granules. The continuously distributed oxides display relatively high contents of Si and Al (point 4 in Supplementary Table 3), while the granular distributed oxides contain relatively high levels of Ti and Al (point 6 in Supplementary Table 3). Notably, it does not peel after 200 h of cyclic oxidation, which suggests it maintains a protective capacity.
The thickness of the unpeeled oxide scale on the bare Ti45Al8.5Nb alloy is approximately 12.1 μm (Fig. 11a). Based on the XRD pattern (Supplementary Fig. 5a) and EDS line scan result (Fig. 11b), it is evident that the outermost layer is predominantly composed of Al2O3. The EDS point analysis also shows this layer is Al enriched (point 1 in Table 5). The second outer layer exhibits high concentrations of Ti, N, and O, indicating a mixture layer comprising TiN and TiO2 (point 2 in Table 5). Beneath these two layers, mixed oxide layers consisting of Al2O3 and TiO2 are observed. Notably, the oxide scale shows clear evidence of Nb diffusion, suggesting the incorporation of Nb as a substitutional atom for Ti in TiO2.
Figure 11d presents the sectional BSE and EDS visuals of the SiO2 coating after 200 h of cyclic exposure at 900 °C. The oxide scale of the specimen with SiO2 coating is about 4.8 μm thick, which is much lower than the oxide layer thickness of the Ti45Al8.5Nb alloy after cyclic oxidation under the same condition (Fig. 11a). Throughout the cyclic oxidation process, Al diffuses into the SiO2 coating, resulting in the formation of an Al2O3 and SiO2 mixture oxide scale (point 4 in Table 5). This oxide scale provides effective protection for the underlying alloy substrate. Additionally, the oxidation of Nb and Ti occurs beneath the Al2O3 and SiO2 mixture oxide scale, resulting in the formation of a (Ti, Nb)O2-rich layer (point 5 in Table 5). This (Ti, Nb)O2-rich layer exhibits higher density than TiO2, thereby offering enhanced protection to the substrate9,10. Between the above oxide layer and the alloy matrix is a TiN layer according to EDS analyses (Fig. 11e, f).
The bond strength and adhesion of the oxide scale to the substrate were evaluated using a scratch test (Fig. 12). In the case of the bare specimen, the voltage drops when the applied load is below 4.2 N (Fig. 12a), indicating the initial failure of the oxide layer. Conversely, the SiO2-coated specimen can withstand a load of approximately 16–19 N (Fig. 12c). Examination of the surface morphology of the scratches reveals that the oxide scale on the bare specimen has peeled off adjacent to the scratch (Fig. 12b1). However, the scratches on the SiO2-coated specimen do not exhibit significant peeling (Fig. 12d1). This demonstrates that the oxide layer formed after 200 h of cyclic oxidation on the SiO2-coated specimen exhibits improved adhesion to the substrate.
Scratch test results (a, c) and BSE images of the scratches (b, d) for bare Ti45Al8.5Nb (a, b) and SiO2 coatings (c, d) after cyclic oxidation at 900 °C for 200 h. The results of three independent tests are given in (a) and (c). The elements distribution mapping is derived from the rectangular region marked in the corresponding BSE images.
Discussion
Protective mechanism of SiO2 coating
In TiAl compositions with elevated Nb concentrations, Nb acts as a pentavalent cation, supplanting Ti4+ within the TiO2 structure. Consequently, the emergence of the (Ti, Nb)O2 phase confers enhanced compactness compared to monolithic TiO2 layers. Furthermore, the doping of Nb in the alloy can reduce the critical Al content used for the formation of outer Al2O3. In addition, the presence of Nb2Al or NbAl3 compounds has a considerable inhibition impact on the formation of the Z phase that can be transformed into Al2O3 and TiO2 mixed oxides during the high-temperature oxidation process9,52,53.
Figure 13 provides a schematic representation detailing the transformation of the Ti45Al8.5Nb alloy and SiO2 coating when subjected to thermal exposure in an air environment at elevated temperatures. After 100 h of isothermal oxidation at 900 °C, the surface of the bare Ti45Al8.5Nb alloy exhibited a continuous, albeit irregular, thin layer of Al2O3 (Fig. 13a). The oxidation kinetic curve (curve1 in Fig. 2a) reveals a rapid weight gain of the bare alloy during the initial 10 h of oxidation, followed by a significant slowdown. This indicates the early formation of an Al2O3 film, and this Al2O3 formation was due to Nb increasing the activity of Al54. However, this Al2O3 layer alone does not provide sufficient protection. Beneath the Al2O3 layer, a TiO2 layer forms. Furthermore, an enrichment layer containing N and Ti is observed beneath the TiO2 layer, corresponding to TiN based on XRD pattern analysis (Fig. 3).
The SiO2 coating exhibits excellent thermal stability and low atom and ion diffusion rates31,36,55. By applying a SiO2 coating to the Ti45Al8.5Nb alloy, the substrate is directly protected without experiencing severe initial oxidation (Fig. 13b). The dense structure of SiO2 coating inhibits the diffusion of O in the air to the substrate. This is why there is no initial rapid growth phase in the kinetic curve of the SiO2 coating (curve 3 in Fig. 2). Additionally, SiO2 coating exhibits good thermal stability. This is reflected in the fact that the coating remains intact without cracking after 200 h of cyclic oxidation (Supplementary Fig. 6b). The oxide scale on bare Ti45Al8.5Nb alloy, by contrast, has already shown extensive peeling (Supplementary Fig. 6a). Moreover, the SiO2 coating exhibits good adhesion to the Ti45Al8.5Nb alloy. The scratch test indicates that the critical load of the oxide scale on SiO2 coating is 4–5 times that of the oxide scale on bare Ti45Al8.5Nb alloy. In addition, the dense transition layer formed due to the interdiffusion between SiO2 and the substrate provides the coating with good bonding force and oxidation resistance. At the same time, Nb diffuses into the oxide layer to form new phases, which will be discussed in depth later.
Interface reaction between the SiO2 coating and Ti45Al8.5Nb alloy
As is pointed out in the schematic diagram (Fig. 13b). In high-temperature oxidation, Si diffuses from the coating to the substrate, while Ti, Al, and Nb diffuse in the opposite direction. The three-layer structure of the oxide layer is based on the following reactions48,49,56:
The calculated Gibbs free energy varies with temperature for these four reactions are shown in Fig. 14. The standard Gibbs free energy changes for reactions (1) and (2) at 900 °C are −703 kJ mol−1 and −1115 kJ mol−1, respectively. The standard Gibbs free energy changes for reactions (3) and (4) at 900 °C to produce 1 mole of oxide are −727 kJ mol−1 and −1308 kJ mol−1, respectively. Initially, SiO2 coatings are not completely dense due to cracks formed during the preparation process. When a small amount of oxygen passes through the SiO2 coating, the TiO2 layer initially grows beneath the SiO2 coating due to its lower growth activation energy compared to Al2O357,58,59. Simultaneously, Ti diffuses into and reacts with the SiO2 coating, resulting in the creation of TiO2 and Ti5Si3, as indicated by reaction (1). And since the solubility of Al in silicide is low, Ti5Si3 acts as a diffusion barrier layer for Al, and then Al2O3 is formed near the Ti5Si3 layer60. Subsequently, Ti and Al further diffuse and react with the SiO2 coating, resulting in the formation of a Ti5Si3 + Al2O3 layer, as depicted by reaction (2). Furthermore, since Ti consumes some of the diffused oxygen at the interface, the low partial pressure of oxygen promotes the formation of Al2O361. The Ti5Si3 + Al2O3 layer above the Ti45Al8.5Nb substrate exhibits excellent thermal stability and low diffusion rates for atoms and ions, providing robust protection for the alloy substrate62,63. HR-TEM analysis confirms the presence of the Nb2Al in the Ti5Si3 and Al2O3 mixture layer, as shown in Fig. 9a4. Additionally, elements distribution mapping demonstrates Nb enrichment within the Ti5Si3 and Al2O3 mixture layers. It is generally accepted that the enhanced oxidation resistance of Nb in TiAl-based alloys is also attributed to Nb2Al as a reaction product, which inhibits the formation of the Z phase10. After this, Nb will continue to diffuse toward the outer layer. Notably, Nb enrichment is also observed in the TiO2 layer according to EDS mappings, which potentially reduces oxygen vacancy concentration and contributes to the formation of a denser structure. However, the diffusion of Nb stops here, and its presence is almost undetectable in the SiO2 coating due to the high density of the SiO2 structure.
Influence of electrodeposition parameters
Increasing the electrodeposition time or current density results in a thicker SiO2 coating. As shown in Supplementary Fig. 2, the SiO2 particles also exhibit increased size. However, cracks may occur in the coating during the drying process due to the evaporation of the solvent. Additionally, thermal stress during vacuum sintering after electrodeposition can further contribute to crack formation. If these cracks are not sufficiently deep, they can self-heal during isothermal oxidation due to the fluidity of the SiO2 coating at 900 °C64. However, when the SiO2 coating thickness is too high, such as exceeding ~5 μm, complete self-healing becomes challenging. Consequently, a dense SiO2 coating cannot be formed, limiting its ability to protect the substrate from oxidation. The oxidation kinetics reveal that the SiO2 coating, electrodeposited at 3 mA cm−2 for 300 s, exhibits a mass increase of 0.95 mg cm−2, surpassing the mass gain of the uncoated sample (0.7 mg cm−2). This can be attributed to the non-dense coating failing to provide adequate substrate protection while simultaneously impeding the formation of Al2O3 by preventing aluminum diffusion to the surface. As a result, internal oxidation occurs primarily, leading to the formation of alternating oxide bands.
Methods
Materials and coating preparation
Ti45Al8.5Nb (at.%) was cut into flakes from a homogenized ingot which was cast by a vacuum furnace. Utilizing 60 SiC grit abrasive sheets, the samples were ground and then rinsed with deionized water after being cleansed with anhydrous ethanol. The BSE image and XRD pattern of this alloy are given in Supplementary Fig. 1. It is mainly composed of γ + α2 biphasic lamellar structure, with trace amounts of β0 phase that falls below the detectable threshold by XRD.
Tetraethoxysilane (TEOS) was used as the precursor for the electrodeposition of SiO2, and the electrolyte preparation process was shown in our previous work36. In the electrodeposition procedure, two graphite pieces spaced 10 mm apart served as the anodes, while the Ti45Al8.5Nb alloy was suspended between them, acting as the cathode. SiO2 coating was electrodeposited under different current densities (0.5 mA cm−2, 1 mA cm−2, 1.5 mA cm−2, 2 mA cm−2, 2.5 mA cm−2, and 3 mA cm−2) and duration time (100 s, 300 s, and 500 s). The obtained SiO2 coating was rinsed with alcohol and then vacuum sintered in a vacuum tube furnace. Use a vacuum pump to evacuate the tube furnace until the pressure is less than 0.1 Pa, then heat it to 800 °C at a heating rate of 5 °C/min, keep it at this temperature for 5 h, then cool it to room temperature in the furnace and take it out.
Oxidation experiments
To evaluate the oxidation performance, the SiO2 coatings were subjected to oxidation in a furnace at 900 °C for 100 h (KSY-12-16, Shanghai Tianye Experimental Electric Furnace Factory). The specimens were set in an Al2O3 crucible and then retrieved and cooled in ambient air at specific time intervals. And then, they were measured on an electronic balance with an accuracy of 0.1 mg. Next, the specimens were returned to the furnace. In a cyclic oxidation oven, the cyclic oxidation experiment was conducted (KJ-X13, Luoyang Keju Furnace Industry Co., Ltd.). In each cycle, the specimen underwent a 50-min air exposure at 900 °C, followed by a 10-min air cooling. Three parallel specimens were employed for the isothermal and cyclic oxidation test.
Sample characterizations
The phase composition of the Ti45Al8.5Nb alloy, as well as the chemical composition of each specimen after isothermal oxidation and cyclic oxidation, were determined by XRD(&&TD-3500, Dandong Tongda Science and Technology Co., Ltd.) equipped with Cu Kα radiation (λ = 0.154056 nm) at 40 kV and 30 mA. A SEM (XL, Thermo Scientific Phenom) was used to observe the micro-morphology and elemental analysis of the specimens before and after oxidation. The interface interaction details between the SiO2 coating and Ti45Al8.5Nb substrate were analyzed using a transmission electron microscope (TEM, TalosS-FEG, Thermo Fisher Scientific) at the interface. The FIB workstation was used to create thin-layer specimens via the in situ lift-out technique.
A scratch tester was employed to assess the bond strength and adhesion between the oxide scale and the substrate (WS-2005, China). The scratch meter operated in conductive mode, establishing an electrical circuit between the probe and the base metal. When the electrical circuit is closed, it indicates that the probe has already destroyed the oxide scale and reached the alloy substrate. Therefore, a reduction in voltage drop is indicative of the failure of the oxide scale.
Data availability
Data supporting the findings of this study are available from the corresponding author and the first author upon reasonable request.
References
Appel, F., Oehring, M. & Wagner, R. Novel design concepts for gamma-base titanium aluminide alloys. Intermetallics 8, 1283–1312 (2000).
Clemens, H. & Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 15, 191–215 (2013).
Duan, B. et al. History and development of γ-TiAl alloys and the effect of alloying elements on their phase transformations. J. Alloy. Compd. 909, 164811 (2022).
Laska, N., Swadźba, R., Nellessen, P., Helle, O. & Anton, R. Oxidation behavior of Ti2AlC MAX phase-based coating on a γ-TiAl alloy TiAl48-2–2 produced by DC magnetron sputtering. Surf. Coat. Technol. 480, 130601 (2024).
Dutta, A., Weisheit, A. & Dutta Majumdar, J. Effect of silicon on the kinetics and mechanisms of cyclic oxidation of titanium aluminide (Ti45Al5Nb0.5Si) developed by laser direct energy deposition. Corros. Sci. 231, 111963 (2024).
Rahmel, A., Schütze, M. & Quadakkers, W. Fundamentals of TiAl oxidation—a critical review. Mater. Corros. 46, 271–285 (1995).
Lin, X. et al. Oxidation properties of Ti–47.5Al–2.5V–1.0Cr–0.2Zr alloy at 950–1050 °C. Mater. Sci. Technol. 39, 1322–1336 (2023).
Genc, O. & Unal, R. Development of gamma titanium aluminide (γ-TiAl) alloys: a review. J. Alloy. Compd. 929, 167262 (2022).
Yoshihara, M. & Miura, K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics 3, 357–363 (1995).
Lin, J. et al. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 19, 131–136 (2011).
Li, D., Zhang, G., Lu, G., Wang, J. & Liu, C. Optimizing high-temperature oxidation behaviors of high-Nb-containing TiAl alloys by addition of boron. Corros. Sci. 177, 108971 (2020).
Zhao, P. et al. Insights into the role of W/B alloying on high-temperature oxidation behavior of Ti42Al5Mn alloy. J. Mater. Sci. Technol. 178, 188–200 (2024).
Xiang, L. L., Zhao, L. L., Wang, Y. L., Zhang, L. Q. & Lin, J. P. Synergistic effect of Y and Nb on the high temperature oxidation resistance of high Nb containing TiAl alloys. Intermetallics 27, 6–13 (2012).
Gao, Z., Hu, R., Zou, H., Zhou, M. & Luo, X. Insight into the Ta alloying effects on the oxidation behavior and mechanism of cast TiAl alloy. Mater. Des. 241, 112941 (2024).
Xiao, S. et al. Origin of the enhanced creep properties of high Nb containing TiAl alloys with the addition of C and Y2O3. Mater. Sci. Eng. A 910, 146902 (2024).
Gui, W. et al. High Nb–TiAl-based porous composite with hierarchical micro-pore structure for high temperature applications. J. Alloy. Compd. 744, 463–469 (2018).
Pan, Y. et al. Effect of Sn addition on the high-temperature oxidation behavior of high Nb-containing TiAl alloys. Corros. Sci. 166, 108449 (2020).
Li, Z.-X. et al. The long-term isothermal oxidation and cyclic oxidation performance of anodized Ti48Al2Nb2Cr alloy. Corros. Sci. 199, 110200 (2022).
Bauer, P.-P., Swadźba, R., Klamann, L. & Laska, N. Aluminum diffusion inhibiting properties of Ti5Si3 at 900 °C and its beneficial properties on Al-rich oxidation protective coatings on γ-TiAl. Corros. Sci. 201, 110265 (2022).
Li, Z.-X., Liu, Y.-H., Liu, H.-J., Wu, L.-K. & Cao, F.-H. Effect of magnetron sputtered Al coating and anodization on the hot corrosion performance of Ti48Al2Nb2Cr alloy. Mater. Today Commun. 36, 106524 (2023).
Liu, X. et al. Isothermal oxidation behaviour of TiAl alloys prepared by spark plasma sintering with the addition of Gd under water vapour at 900 °C. Intermetallics 153, 107796 (2023).
Wang, Y. et al. Improving high temperature oxidation resistance of TiAl alloy via hierarchical Ti5Si3–Ti2AlC precipitation strategy. Corros. Sci. 228, 111834 (2024).
Crespo-Villegas, J., Cavarroc, M., Knittel, S., Martinu, L. & Klemberg-Sapieha, J. E. Protective TixSiy coatings for enhanced oxidation resistance of the ɣ-TiAl alloy at 900 °C. Surf. Coat. Technol. 430, 127963 (2022).
Yang, L. et al. Oxidation behavior of the AlN coatings on the TiAl alloy at 900 °C. Corros. Sci. 211, 110891 (2023).
Zhang, X. et al. Microstructure evolution and thermal shock properties of PEO coatings on a TiAl alloy. Surf. Coat. Technol. 454, 129208 (2023).
Kagerera, S. et al. Oxidation protection of TNM alloys with Al-rich γ-TiAl-based coatings. J. Alloy. Compd. 969, 172343 (2023).
Zhang, Y. et al. High temperature oxidation resistance of TNM alloy coated with/without 8YSZ/NiCoCrAlY thermal barrier coatings. Appl. Surf. Sci. 611, 155704 (2023).
Pan, Y. et al. Thermal insulation performance and thermal shock resistance of plasma-sprayed TiAlCrY/Gd2Zr2O7 thermal barrier coating on γ-TiAl alloy. Surf. Coat. Technol. 468, 129715 (2023).
Li, Y. et al. Microstructure evolution and cyclic oxidation performance of Cr2AlC as active diffusion barrier for NiCrAlY coating on TiAl alloy. Corros. Sci. 226, 111696 (2024).
Han, D. et al. Isothermal oxidation resistance and microstructure evolution of VPS-TiAlCrY coating on TiAl single crystals at 1100–1200 °C. Corros. Sci. 208, 110664 (2022).
Xu, S. et al. High temperature oxidation resistance of Ti-5553 alloy with electro-deposited SiO2 coating. Mater. Chem. Phys. 275, 125306 (2022).
Yu, C., Zhu, S., Wei, D. & Wang, F. Amorphous sol-gel SiO2 film for protection of Ti6Al4V alloy against high temperature oxidation. Surf. Coat. Technol. 201, 5967–5972 (2007).
Taniguchi, S., Shibata, T. & Katoh, N. Improvement in the high-temperature oxidation resistance of TiAl by sol-derived SiO2 coating. J. Jpn. I. Met. 57, 666–673 (1993).
Teng, S., Liang, W., Li, Z. & Ma, X. Improvement of high-temperature oxidation resistance of TiAl-based alloy by sol–gel method. J. Alloy. Compd. 464, 452–456 (2008).
Zhang, X., Zhao, S., Gao, C. & Wang, S. Amorphous sol-gel SiO2 film for protection of an orthorhombic phase alloy against high temperature oxidation. J. Sol. Gel Sci. Technol. 49, 221–227 (2009).
Wu, L.-K. et al. Enhanced high temperature oxidation resistance for γ-TiAl alloy with electrodeposited SiO2 film. Corros. Sci. 140, 388–401 (2018).
Liu, L. & Mandler, D. Sol-gel coatings by electrochemical deposition. The Sol-Gel Handbook 373–414 (Wiley-Blackwell, 2015).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Gong, X. et al. Effect of Mo on microstructure and oxidation of NiCoCrAlY coatings on high Nb containing TiAl alloys. Appl. Surf. Sci. 431, 81–92 (2018).
Ebach-Stahl, A. & Fröhlich, M. Lifetime study of sputtered PtAl coating on γ-TiAl with and without TBC topcoat at high temperatures. Surf. Coat. Technol. 377, 124907 (2019).
Fröhlich;, M., Braun, R. & Leyens, C. Oxidation resistant coatings in combination with thermal barrier coatings on γ-TiAl alloys for high temperature applications. Surf. Coat. Technol. 201, 3911–3917 (2006).
Yang, X. et al. Influence of solvent and electrical voltage on cathode plasma electrolytic deposition of Al2O3 antioxidation coatings on Ti–45Al–8.5Nb alloys. Metals 8, 308 (2018).
Zhang, K., Zhang, T. & Song, L. Oxidation behavior of a high-Nb-containing TiAl alloy with multilayered thermal barrier coatings. J. Therm. Spray. Techn. 27, 999–1010 (2018).
Jiang, Z. et al. Favorable deposition of γ-Al2O3 coatings by cathode plasma electrolysis for high-temperature application of Ti–45Al–8.5Nb alloys. Surf. Coat. Technol. 333, 187–194 (2018).
Gao, J., He, Y. & Gao, W. Electro-codeposition of Al2O3–Y2O3 composite thin film coatings and their high-temperature oxidation resistance on γ-TiAl alloy. Thin Solid Films 520, 2060–2065 (2012).
Yang, Y. et al. Improved oxidation resistance of γ-TiAl intermetallics by sputtered Ni + CrAlYHfSiN composite coating. Corros. Sci. 187, 109510 (2021).
Zheng, K. et al. Microstructure, oxidation behavior and adhesion of a CoNiCrAlTaY coating deposited on a high Nb–TiAl alloy by plasma surface metallizing technique. Vacuum 179, 109494 (2020).
Cockeram, B. V. & Rapp, R. A. The kinetics of multilayered titanium-silicide coatings grown by the pack cementation method. Metall. Mater. Trans. A 26, 777–791 (1995).
Li, W. et al. SiO2–Al2O3–glass composite coating on Ti–6Al–4V alloy: oxidation and interfacial reaction behavior. Corros. Sci. 74, 367–378 (2013).
Jiang, H., Hirohasi, M., Lu, Y. & Imanari, H. Effect of Nb on the high temperature oxidation of Ti-(0–50 at.%)Al. Scr. Mater. 46, 639–643 (2002).
Seifert, H., Kussmaul, A. & Aldinger, F. Phase equilibria and diffusion paths in the Ti–Al–O–N system. J. Alloy. Compd. 317, 19–25 (2001).
Chen, G., Sun, Z. & Zhou, X. Oxidation of intermetallic alloys in Ti–Al–Nb ternary system. Corrosion 48, 939–946 (1992).
Lu, W. et al. (S)TEM study of different stages of Ti–5Al–8Nb–0.2W–0.2B–0.02Y alloy oxidation at 900 °C. Corros. Sci. 50, 978–988 (2008).
Lu, W., Chen, C., He, L., Xi, Y. & Wang, F. Effect of niobium on the oxidation behavior of TiAl. J. Mater. Res. 22, 1486–1490 (2007).
Wu, L.-K. et al. High temperature oxidation resistance of γ-TiAl alloy with pack aluminizing and electrodeposited SiO2 composite coating. Corros. Sci. 146, 18–27 (2019).
Liao, Y. et al. Self-healing metal-enamel composite coating and its protection for TiAl alloy against oxidation under thermal shock in NaCl solution. Corros. Sci. 167, 108526 (2020).
Dai, J., Zhu, J., Chen, C. & Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: a review. J. Alloy Compd. 685, 784–798 (2016).
Rahmel, A. & Spencer, P. Thermodynamic aspects of TiAl and TiSi2 oxidation: the Al–Ti–O and Si–Ti–O phase diagrams. Oxid. Met. 35, 53–68 (1991).
Li, Y., Dai, J. & Song, Y. Research progress of first principles studies on oxidation behaviors of Ti–Al alloys and alloying influence. Metals 11, 985 (2021).
Crespo-Villegas, J., Cavarroc, M., Knittel, S., Martinu, L. & Klemberg-Sapieha, J. E. Self-organized multilayer structure in magnetron sputtered SiPt reservoirs on γ-TiAl alloy induced by high-temperature annealing and oxidation. Surf. Coat. Technol. 447, 128865 (2022).
Legzdina, D., Robertson, I. M. & Birnbaum, H. K. Oxidation behavior of a single phase γ-TiAl alloy in low-pressure oxygen and hydrogen. Acta Mater. 53, 601–608 (2005).
Li, X. Y., Taniguchi, S., Matsunaga, Y., Nakagawa, K. & Fujita, K. Influence of siliconizing on the oxidation behavior of a γ-TiAl based alloy. Intermetallics 11, 143–150 (2003).
Huang, J., Zhao, F., Cui, X., Wang, J. & Xiong, T. Long-term oxidation behavior of silicon-aluminizing coating with an in-situ formed Ti5Si3 diffusion barrier on γ-TiAl alloy. Appl. Surf. Sci. 582, 152444 (2022).
Ding, J. et al. Enhanced high-temperature tensile property by gradient twin structure of duplex high-Nb-containing TiAl alloy. Acta Mater. 161, 1–11 (2018).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51971205, 52271084), and Guangdong Basic and Applied Basic Research Foundation (2021B1515020056).
Author information
Authors and Affiliations
Contributions
Lian-Kui Wu performed the conception and design of the high-temperature oxidation of SiO2 coating. Liang-Liang Wu, Hao-Jie Yan, and Tao Fan performed the acquisition, analysis, or interpretation of the data. Qing-Qing Sun performed the microscopy of the coating. Lian-Kui Wu and Fa-He Cao supervised this project and the final approval of the completed version. All authors discussed and contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41529_2024_511_MOESM1_ESM.pdf
Supplementary Fig. 1, Supplementary Fig. 2, Supplementary Fig. 3, Supplementary Fig. 4, Supplementary Fig. 5, Supplementary Fig. 6, Supplementary table 1, Supplementary table 2, Supplementary table 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, LL., Yan, HJ., Fan, T. et al. The oxidation behavior and interfacial reaction between SiO2 coating and Ti45Al8.5Nb alloy. npj Mater Degrad 8, 96 (2024). https://doi.org/10.1038/s41529-024-00511-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-024-00511-4