Abstract
The dynamic disulfide linkage plays a vital role in various biological processes as well as drugs and biomaterials. The conversion of thiols to their corresponding disulfides is a hallmark of sulfur chemistry, but notoriously difficult to control. Achieving optimal reactivity and selectivity continues to pose significant challenges. Here, we describe a click chemistry for disulfide formation from thiols in both batch and flow-mode using SO2F2, which display exceptional selectivity toward disulfide formation through an effective nucleophilic substitution cascade. This reaction’s unique characteristics satisfy the stringent click-criteria with its high thermodynamic driving force, straightforward conditions, wide scope, quantitative yields, exceptional chemoselectivity, and non-chromatographic purification process. The modular synthesis of symmetrical, unsymmetrical, cyclic and polydisulfides is demonstrated, along with the formation of disulfide cross-linked hydrogels.
Similar content being viewed by others
Introduction
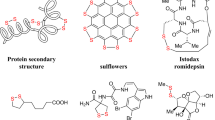
In nature, disulfide bonds are crucial for protein folding and stability1, as well as a prevalent moiety in drugs and biomaterials (Fig.1). Furthermore, the dynamic nature of disulfide bonds renders them attractive reversible linkages for on-demand drug delivery2,3,4,5,6,7,8,9,10,11. Recently, biodegradable cell-penetrating poly(disulfide)s have been employed to deliver drugs, nucleic acids, and proteins into cells via thiol-mediated uptake pathways (Fig. 1B)4,5. Further, antibody-drug conjugates (ADCs) have emerged as a significant class of cancer therapeutics, with disulfides used as the predominant chemically cleavable motifs in ADC linkers (Fig. 1C)6. Disulfide-containing polymers, owing to their inherent biodegradability and biocompatibility, also serve as biomaterials and are extensively utilized in tissue engineering and wound healing applications (Fig. 1D)7,8,9,10,11. Considering the importance of disulfide linkages in drugs, natural products, and biomaterials, it is imperative to develop highly efficient, selective, and biocompatible synthetic methodologies for their construction.
Thiol-disulfide exchange serves as the basis for most in vivo enzyme-catalyzed disulfide linkage formation process12. As for the chemical synthesis of disulfides, using thiols as the precursors is the most straightforward route, because a vast number of thiols are readily available. Converting thiols into disulfides can be accomplished by many oxidants2,13,14,15,16,17,18,19,20,21,22,23. However, high reactivity and efficiency cannot be achieved simultaneously by any known oxidants (Fig. 2A). The unsatisfactory selectivity was exemplified by the formation of over-oxidized by-products and the oxidation of non-target functional group when strong oxidants and/or excessive oxidants were used2,13. In addition, methods for the selective cross-coupling of two different thiols are rare18,19,20,21,22. Recently, Jiang’s group reported robust polysulfurating and bilateral disulfurating reagents for the synthesis of unsymmetrical disulfides24,25,26. Conversely, the absence of an efficient oxidizing agent has rendered the oxidation process of tertiary thiols, as well as the oxidative polymerization of dithiols, challenging to achieve2,13. In fact, valuable poly(disulfide)s have to be prepared from ring-opening thiol-disulfide exchange polymerization or via polymerization of monomers that already contain disulfide moiety7. Due to the lack of reliable synthetic approach, many potentially valuable disulfide bond containing small molecules or polymers are yet to be explored. Furthermore, the toxicity of many of the oxidants or catalysts have made the formation of disulfide under physiological conditions impossible.
Click chemistry, established by Sharpless and colleagues in 2001, requires reactions to be highly selective, efficient, simple and produce harmless products27. Over the last two decades, only a handful of essential click reactions have emerged that enable the formation of reliable connectivity with unparalleled efficiency. Among these reactions are the sulfur (VI)- fluoride exchange (SuFEx) processes developed by Sharpless, Moses, Dong, Jiang and colleagues28,29,30,31,32,33,34,35,36,37. These click chemistries have quickly impacted fields from organic synthesis and material science, to chemical biology and drug discovery.
Herein, we present a SO2F2 mediated click reaction that enables modular synthesis of disulfides from thiols via a nucleophilic substitution cascade.
Results
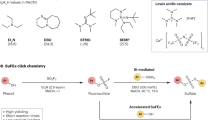
Development of SO2F2 mediated click chemistry for efficient thiol-to-disulfide conversion
The key to developing the click chemistry for disulfide formation is a highly reactive and selective reagent, which effectively converts thiols into disulfides without oxidizing the products and non-target functional groups. As a high valent sulfur reagent, SO2Cl2 is effective for converting primary and secondary thiols into disulfides. However, its low chemoselectivity and high reactivity towards hydrolysis and nucleophilic reaction with non-target functional groups have limited its applications. In contrast, its analogy SO2F2, a gaseous SVI compound known as a fumigant and produced on a million-kilogram scale annually38, exhibits remarkable stability and can persist in water at 150 °C39. The S-F bond is much stronger compared to S-Cl bond, and its cleavage is almost exclusively heterolytic with the formation of fluoride ion40. Consequently, SO2F2 resists reduction by most known reductants due to the high kinetic barrier. For example, triphenylphosphine, a widely used reductant41, can be oxidized by sulfuryl chloride rapidly at r.t42, but the reaction between sulfuryl fluoride and triphenylphosphine is very slow even at elevated temperature (calculated barrier, 43.5 kcal/mol; Fig. 2B)43. Additionally, we have established that other readily oxidizable organic compounds, including 1,2-bis(diphenylphosphino)ethane, phenylpropyl aldehyde and 4-methylbenzaldehyde, exhibit stability in the presence of an excess of SO2F2. Furthermore, our experiments demonstrate that ULTRANOX™ 626, a commercial antioxidant, also resists oxidation by SO2F2, underscoring the remarkable reductive stability of this reagent (see SI 14 for details).
In a preliminary study, we observed that SO2F2 can quickly reacted with a thiol through a nucleophilic substitution cascade (Fig. 2B, C). In the reaction between thiol and SO2F2, the high thermodynamic driving force, calculated 64.4 kcal/mol, renders the reaction “spring-loaded” for a unidirectional trajectory (Fig. 2C). During the first nucleophilic substitution step (1 → 1’, Fig. 2C), SO2F2 was attacked by thiol under basic conditions, resulting in intermediate 1’ (calculated barrier, 11.9 kcal/mol). In the second step, the highly reactive intermediate 1’ was attacked by an additional molecule of thiol, yielding disulfide as the final product. We also validated both experimentally and computationally that the reaction between thiol and SO2F2 possesses a considerably lower activation energy in comparison to the reaction between SO2F2 and other reductant (Fig. 2B), such as triphenylphosphine, (see SI 14 for details). Owing to the distinctive mechanism of the thiol-to-disulfide conversion by SO2F2, this reaction exhibits exceptional efficiency and selectivity.
After recognizing the potential of SO2F2 as a suitable reagent for the thiol-to-disulfide conversion, we initiated the optimization of reaction conditions (Tables S1–3, S8 and S11). Our results indicated that the reaction between thiols and SO2F2 required only simple conditions with weak base and exhibited insensitivity towards the reaction medium and oxygen. This reaction proceeded well at room temperatures. Both organic base, such as Et3N, and inorganic base, for instance Na2CO3 were appropriately capable to trigger reaction. Excellent yields have been obtained in both water and organic solvents, like CH3CN. Given that only inoffensive fluoride salt was formed as the byproduct, the product can be purified utilizing simple nonchromatographic method. Apart from SO2F2 (I) gas, a range of solid and liquid surrogates to SO2F2, namely, sulfamoyl fluorides II, III and IV, sulfonimidoyl fluoride V, sulfonyl fluorides VI and VII, and fluorosulfate VIII could all successfully convert thiols into the corresponding disulfides, with varied reactivity. Based on extensive experimentations, their order of reactivity was as follows: I ≈ II ≈ III > V ≈ VI ≈ VII > IV > VIII.
Given that sulfonyl fluoride VI exhibit lower reactivity compared to SO2F2, we attempted to isolate the intermediate 1’a formed in the reaction between VI and 1a to conduct control experiments. However, direct isolation of the intermediate from the reaction was unsuccessful. Nevertheless, we successfully synthesized the intermediate via the reaction between 1a and 4-methylbenzenesulfonohydrazide. With the synthesized intermediate in hand, we tested the reaction between 1’a and 1a without a base (eq 1), and the results showed that only the starting materials were recovered, indicating that a base is essential for the second step of the reaction (1’ to 2, Fig. 2C). Subsequently, we tested the reaction between 1a and VI without a base (eq 3), and similarly, only the starting materials were recovered with no formation of intermediate 1’a, suggesting that a base is also crucial for the first step (1 to 1’, Fig. 2C). Next, in the presence of a base, we repeated both experiments, but the reaction was quenched after 30 seconds. The reaction between 1’a and 1a yielded >98% of 2a within 30 seconds (eq 2), indicating that the second step proceeds very rapidly in the presence of a base. Conversely, the reaction between 1a and VI in the presence of a base resulted in only 29% yield of 2a (eq 4), with the major mass-balance being unreacted 1a, indicating that the first step is slower than the second step and constitutes the rate-determining step of the reaction.
Having established this uniquely selective method for disulfide synthesis, we next investigated its applicability in diverse synthetic contexts and reaction media (Figs. 2–4).
A Synthesis of symmetrical disulfide. B Synthesis of unsymmetrical disulfide. C Synthesis of cyclic disulfides. Reaction conditions: a The results were the same for 1 h and 12 h. b BTMG was used instead of Et3N; c R1SH (3 equiv.), CH3CN; d R1SH (3 equiv.), CH3CN/Borax (1:1); e R1SH (2 equiv.), CH3CN; f R1SH (1 equiv.), CH3CN.
Modular synthesis of disulfides
This method was applied in the synthesis of symmetric, unsymmetrical and cyclic disulfides using SO2F2/Et3N in CH3CN (Fig. 3). The experimental findings revealed that this reaction exhibited wide scope, quantitative yields and excellent chemoselectivity. All the 31 aliphatic and aromatic thiols surveyed were efficiently converted into the corresponding symmetric disulfides with excellent yields of ≥98% (Fig. 3A), including traditionally challenging substrates such as secondary and tertiary thiols (2ab, 2ac, 2ad). This method exhibited remarkable chemoselectivity and accommodated an array of functional groups, including aniline (2 l, 2r), carboxylic acid (2t, 2ae), ketone (2ac), halide (2c, 2d, 2i, 2j, 2k, 2p, 2q, 2 s), amide (2n, 2ae) and heterocycle (2 u, 2 v, 2w, 2x). Although phenol could react with SO2F2, 2o underwent selective reaction on the thiol group while precluding any undesired interference from the phenol. This can be attributed to the fact that the reactions between phenol and SO2F2 generally necessitate distinct reaction conditions. For example, the reaction between phenols and SO2F2 was typically triggered by 1.5 equiv. of Et3N29. In contrast, the reaction of 2 l or 2o with SO2F2 requires 0.3 equiv. of Et3N. Therefore, even when the reaction time of 2 l or 2o with excess SO2F2 was lengthened from 1 h to 12 h, no by-products were observed. Captopril (2ae), a medicine, was also smoothly converted into the corresponding disulfide.
The symmetric disulfide synthesis using representative solid and liquid reagents II - VIII was also studied (Fig. 3A). The results revealed that all those reagents were as effective as SO2F2 for the reaction with benzyl thiols and thiophenols. In the context of aliphatic thiols, particularly tertiary thiols, quantitative yields were not achieved employing those reagents and Et3N, as the residual mass balance consisted of unreacted starting materials. However, by substituting Et3N with a stronger base, 2-tert-butyl-1,1,3,3-tetramethylguanidine (BTMG), the yields can be significantly enhanced. For example, 98% yield of 2ad was obtained by using reagent III and BTMG.
The efficiency of this click reaction in linking two different modules was evaluated through the direct synthesis of unsymmetrical disulfides (Fig. 3B). Using SO2F2 as the reagent, unsymmetrical disulfides were synthesized from the cross-coupling of one aromatic thiol and one aliphatic thiol, two aromatic thiols, and two aliphatic thiols, achieving yields of 60−98%, which were challenging to obtain with other known coupling reagents. In the coupling reactions involving one aromatic thiol and one aliphatic thiol (3c − 3d and 3 f − 3n), high yields >80% could even be achieved using equimolar amounts of the thiols (3k). This is likely due to the relatively higher acidity of aromatic thiols, which are more easily deprotonated under basic conditions and therefore react more rapidly with SO2F2 compared to aliphatic thiols, resulting in the formation of ArSSO2F as the predominant intermediate. The reaction of the intermediate ArSSO2F with aliphatic thiol subsequently produces the desired unsymmetrical disulfides. Despite the similar reactivity of the two starting materials, this method is also effective for the unsymmetrical coupling of two aromatic thiols (3a-3b) or two aliphatic thiols (3e), although 2.0 – 3.0 equiv. of one reactant was required to achieve high yields. In addition, the reaction of 1,3- and 1,4-dithiols (dihydrolipoic acid methyl ester and dithiothreitol) was also examined, which gave five- and six-membered cyclic disulfides (4a and 4b) with 63% and 98% yields, respectively (Fig. 3C).
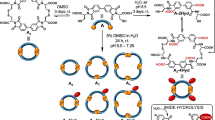
Thiol-to-disulfide click chemistry in water
Upon investigating the scope of this click reaction in organic solvent, we subsequently established its efficacy under aqueous and more complex physiological conditions (Fig. 4). Primarily, for water-soluble substrates, like poly(ethylene glycol) methyl ether thiol (mPEG-SH), water may serve as an appropriate solvent in conjunction with water-soluble inorganic bases. For example, mPEG-SH was converted into 2ah with 98% yield using SO2F2/Na2CO3 in water. Given the importance of organic reactions compatible with biological media for numerous biological and medical applications, we further assessed the reaction in intricate biological media and under physiological pH conditions. Our findings revealed that, in the medium of bovine serum albumin, mPEG-SH could be converted to the corresponding disulfide with a 98% yield, utilizing SO2F2/Na2CO3. Moreover, under physiological pH conditions maintained by a borax buffer, boc-L-cysteine and glutathione were successfully converted to the corresponding disulfides 2af and 2ag, achieving yields of 85% and 98%, respectively, when mediated by SO2F2/Et3N. The unsymmetrical disulfides 3a and 3c can also be synthesized under this aqueous condition (Fig. 3B).
Flow-click chemistry
To enhance the prospective industrial applicability of this reaction and facilitate its large-scale implementation, we have devised a tube-in-tube flow-click chemistry apparatus tailored for the efficient synthesis of disulfides (Fig. 5). In this apparatus, the inner tube was filled with SO2F2, which diffused through the inner tube’s wall into the solution of the thiol and base situated between the inner and outer tubes, thereby triggering the thiol-to-disulfide conversion. The outer tube was fabricated with Teflon, while the inner tube was constructed from a polyvinylidene fluoride microporous hollow fiber membrane. The hollow fiber membrane, an inexpensive and readily accessible semipermeable material, is commonly employed in water treatment processes. Owing to its high specific surface area and exceptional mass-transfer properties, the hollow fiber membrane effectively facilitated the mixing of gaseous SO2F2 within the inner tube and the liquid reaction mixture present in the outer tube. This work represented a unique application of the hollow fiber membrane in gas-liquid flow chemistry44,45. By sealing the inner tube’s terminus with a balloon, the potential leakage of SO2F2 was effectively circumvented. The reactor shown in Fig. 5 has been used in the thiol-to-disulfide conversion of captopril and glutathione which gave >95% yields and a production rate of 5 mmol/h. The potential for further scale-up of this reactor can be efficiently realized by implementing multiple parallel tubes in the design. Additionally, the reactor demonstrates versatility as it is well-suited for the scale-up of other gas-liquid reactions (see SI 16 for details).
The tube-in-tube flow-click chemistry apparatus and its application to large scale disulfide synthesis. Reaction conditions: the solution of thiol and Et3N was pumped into the outer tube at a rate of 1 mL/min after SO2F2 was introduced into the inner tube. The apparatus operated for 1 h at room temperature.
Modular synthesis of poly(disulfide)s
Subsequently, this reaction’s ability to link modular thiols was further demonstrated in the synthesis of polymers (Tables 1 and 2). Traditionally, polymerization of dithiols was very challenging. In this study, the polymerization was investigated using a hydrophobic monomer 5a and a hydrophilic monomer 5b as models. Monomer 5a underwent complete polymerization within a duration of 0.5 h by employing SO2F2/Et3N or reagent II/Et3N in organic solvent (Table 1). In the case of 5b, the utilization of either organic base/solvent or inorganic base/water was feasible (Table 2). It was noteworthy that crucial parameters, including average molecular weight and the polydispersity index (PDI), exhibited a decrease in correlation to the increment of Et3N concentration (entries 1–3, Table 1). Replacing SO2F2 with reagent II resulted in smaller average molecular weight and larger polydispersity index (entry 4, Table 1). Under the aqueous polymerization conditions, the utilization of a stronger base NaOH instead of a weaker base Na2CO3 resulted in a higher average molecular weight and smaller polydispersity index of the polymer (entries 3–4, Table 2). The rapid and controllable polymerization of dithiols accomplished by our click reaction will enable the preparation of various poly(disulfide)s to meet the different requirements for drug delivery and biomaterial synthesis.
In-situ gelation by thiol-to-disulfide click reaction
This thiol-to-disulfide click reaction was applied as a practical cross-linking method for the in-situ gelation of hydrogels (Fig. 6). Hydrogel scaffold, N-acetyl-L-cysteine modified chitosan, was selected for investigation. The results showed that rapid hydrogel formation could be achieved by mixing an aqueous solution of N-acetyl-L-cysteine modified chitosan and Et3N, and a solution of SO2F2 in CH3CN (Fig. 6A). The dynamic time sweep rheological experiment revealed an immediate cross-over of G′ and G″ (Fig. S10), signifying the swift formation of a 3D network through disulfide cross-linkers. When the freshly mixed in-situ gel was applied to porcine skin, a tissue adhesive strength of 42.5 kPa was observed after 16 h, which was comparable to that of other in-situ hydrogels cured by UV radiation (Table S13). In a separate experiment, a colored in-situ gel was applied to fresh porcine skin. Even after stretching and twisting the skin tissue, there were no signs of breakage or detachment of the adhesive hydrogel (Fig. 6B). These findings indicated the potential for future applications of this thiol-to-disulfide click chemistry in the field of biomaterials.
Discussion
In conclusion, we have successfully designed and developed a click reaction for the reliable construction of disulfide linkage from thiols that meets the rigorous criteria of click chemistry. This thiol-to-disulfide click reaction is poised to enable the development of drugs, drug delivery systems, and innovative polymeric biomaterials, paving the way for enhanced therapeutic solutions and material technologies.
Methods
General procedure for the synthesis of symmetrical aliphatic disulfide using SO2F2
To a solution of aliphatic thiol (0.500 mmol, 1.00 equiv.) and Et3N (1.00 mmol, 2.00 equiv.) in CH3CN (0.500 mL), SO2F2 was introduced by needle from a balloon filled with the gas. The reaction mixture was stirred vigorously at r.t for 30 min. Then, excess SO2F2 was quenched by passing it through a solution of thiol and Et3N in CH3CN. The reaction mixture was diluted with EtOAc (30.0 mL), washed with brine (3 × 5.00 mL), and the organic layer were dried over Na2SO4, filtered and evaporated to give the pure product.
General procedure for the synthesis of symmetrical aromatic disulfide using SO2F2
To a solution of aromatic thiol (0.500 mmol, 1.00 equiv.) and Et3N (0.150 mmol, 0.300 equiv.) in CH3CN (0.500 mL), SO2F2 was introduced by needle from a balloon filled with the gas. The reaction mixture was stirred vigorously at r.t for 1 h. Then, excess SO2F2 was quenched by passing it through a solution of thiol and Et3N in CH3CN. The reaction mixture was diluted with EtOAc (30.0 mL), washed with brine (3 × 5.00 mL), and the organic layer were dried over Na2SO4, filtered and evaporated to give the pure product.
General procedure for the synthesis of unsymmetrical disulfide using SO2F2
To a solution of R1SH (0.500−1.50 mmol, 1.00−3.00 equiv.), R2SH (0.500 mmol, 1.00 equiv.), and Et3N (1.00 mmol, 2.00 equiv.) in CH3CN or CH3CN/Borax (1:1) (0.500 mL), SO2F2 was introduced by needle from a balloon filled with the gas. The reaction mixture was stirred vigorously at r.t. After 30 min, excess SO2F2 was quenched by passing it through a solution of thiol and Et3N in CH3CN. The solvent was then removed by rotary evaporation and the crude product was purified by flash chromatography (silica, 0–25% EtOAc/Hexane).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Experimental data as well as characterization data for all new compounds prepared in the course of these studies are provided in the Supplementary Information. The data supporting the findings of this study are available within the main text and the Supplementary Information. Source data are provided in this paper. Data supporting the findings of this manuscript are also available from the authors upon request. Source data are provided with this paper.
References
Fass, D. & Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 118, 1169–1198 (2018).
Wang, M. & Jiang, X. Sulfur–Sulfur Bond Construction. Top. Curr. Chem. 376, 14 (2018).
Jiang, C.-S., Müller, W. E. G., Schröder, H. C. & Guo, Y.-W. Disulfide- and Multisulfide-Containing Metabolites from Marine Organisms. Chem. Rev. 112, 2179–2207 (2012).
Zhang, R., Nie, T., Fang, Y., Huang, H. & Wu, J. Poly(disulfide)s: From Synthesis to Drug Delivery. Biomacromolecules 23, 1–19 (2022).
Laurent, Q. et al. Thiol-Mediated Uptake. JACS Au 1, 710–728 (2021).
Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Bang, E.-K., Lista, M., Sforazzini, G., Sakai, N. & Matile, S. Poly(disulfide)s. Chem. Sci. 3, 1752–1763 (2012).
Fiore, G. L., Rowan, S. J. & Weder, C. Optically healable polymers. Chem. Soc. Rev. 42, 7278–7288 (2013).
Bej, R. & Ghosh, S. Poly(disulfide)s. in Sulfur‐Containing Polymers 367–392 (Wiley, 2021).
Muir, V. G. & Burdick, J. A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 121, 10908–10949 (2021).
Gao, Y., Peng, K. & Mitragotri, S. Covalently Crosslinked Hydrogels via Step‐Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 33, 2006362 (2021).
Riemer, J., Bulleid, N. & Herrmann, J. M. Disulfide Formation in the ER and Mitochondria: Two Solutions to a Common Process. Science. 324, 1284–1287 (2009).
Uemura, S. Oxidation of Sulfur, Selenium and Tellurium. in Comprehensive Organic Synthesis 757–787 (Elsevier, 1991).
Song, J. et al. A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate–Metal Organic Framework for Aerobic Decontamination. J. Am. Chem. Soc. 133, 16839–16846 (2011).
Li, X.-B. et al. Mechanistic Insights into the Interface-Directed Transformation of Thiols into Disulfides and Molecular Hydrogen by Visible-Light Irradiation of Quantum Dots. Angew. Chem. Int. Ed. 53, 2085–2089 (2014).
Bottecchia, C. et al. Batch and Flow Synthesis of Disulfides by Visible-Light-Induced TiO2 Photocatalysis. Chem. Sus. Chem. 9, 1781–1785 (2016).
Sun, Z. et al. A structured catalyst based on cobalt phthalocyanine/calcined Mg–Al hydrotalcite film for the oxidation of mercaptan. Green. Chem. 14, 1909–1916 (2012).
Zhang, J. & Studer, A. Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides. Nat. Commun. 13, 3886 (2022).
Wu, Z. & Pratt, D. A. Radical Substitution Provides a Unique Route to Disulfides. J. Am. Chem. Soc. 142, 10284–10290 (2020).
Oka, M., Katsube, D., Tsuji, T. & Iida, H. Phototropin-Inspired Chemoselective Synthesis of Unsymmetrical Disulfides: Aerobic Oxidative Heterocoupling of Thiols Using Flavin Photocatalysis. Org. Lett. 22, 9244–9248 (2020).
Dou, Y. et al. Reusable cobalt-phthalocyanine in water: efficient catalytic aerobic oxidative coupling of thiols to construct S–N/S–S bonds. Green. Chem. 19, 2491–2495 (2017).
Song, L. et al. Natural gallic acid catalyzed aerobic oxidative coupling with the assistance of Mn(CO3)2 for synthesis of disulfanes in water. Green. Chem. 21, 1432–1438 (2019).
Corma, A., Ródenas, T. & Sabater, M. J. Aerobic oxidation of thiols to disulfides by heterogeneous goldcatalysts. Chem. Sci. 3, 398–404 (2012).
Xiao, X., Xue, J. & Jiang, X. Polysulfurating reagent design for unsymmetrical polysulfide construction. Nat. Commun. 9, 2191 (2018).
Xue, J. & Jiang, X. Unsymmetrical polysulfidation via designed bilateral disulfurating reagents. Nat. Commun. 11, 4170 (2020).
Yu, Q., Bai, L. & Jiang, X. Disulfide Click Reaction for Stapling of S-terminal Peptides. Angew. Chem. - Int. Ed. 62, e202314379 (2023).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Li, S. et al. SuFExable polymers with helical structures derived from thionyl tetrafluoride. Nat. Chem. 13, 858–867 (2021).
Zheng, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. 116, 18808–18814 (2019).
Mortenson, D. E. et al. Inverse Drug Discovery” Strategy To Identify Proteins That Are Targeted by Latent Electrophiles As Exemplified by Aryl Fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018).
Kitamura, S. et al. Sulfur(VI) Fluoride Exchange (SuFEx)-Enabled High-Throughput Medicinal Chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Smedley, C. J. et al. Diversity Oriented Clicking (DOC): Divergent Synthesis of SuFExable Pharmacophores from 2‐Substituted‐Alkynyl‐1‐Sulfonyl Fluoride (SASF) Hubs. Angew. Chem. Int. Ed. 59, 12460–12469 (2020).
Smedley, C. J. et al. Accelerated SuFEx Click Chemistry For Modular Synthesis. Angew. Chem. - Int. Ed. 61, e202112375 (2022).
Zeng, D., Ma, Y., Deng, W. P., Wang, M. & Jiang, X. Divergent sulfur(VI) fluoride exchange linkage of sulfonimidoyl fluorides and alkynes. Nat. Synth. 1, 455–463 (2022).
Zeng, D., Ma, Y., Deng, W. P., Wang, M. & Jiang, X. The Linkage of Sulfonimidoyl Fluorides and Unactivated Alkenes via Hydrosulfonimidoylation. Angew. Chem. - Int. Ed. 61, e202207100 (2022).
Lou, T. S.-B. & Willis, M. C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat. Rev. Chem. 6, 146–162 (2022).
Wiberg, N., Holleman, A. F., & Wiberg, E. The Chalcogen Group. in Inorganic Chemistry 550 (Academic Press, 2001).
Chatgilialoglu, C. Sulfonyl radicals. in The chemistry of sulphones and sulphoxides 1089-1111 (John Wiley & Sons, 1988).
Bellale, E. V., Chaudhari, M. K. & Akamanchi, K. G. A simple, fast and chemoselective method for the preparation of arylthiols. Synth. (Stuttg.) 19, 3211–3213 (2009).
Beddoe, R. H. et al. Redox-neutral organocatalytic Mitsunobu reactions. Science. 365, 910–914 (2019).
Steinkopf, W. Ü. ber Aromatische Sulfofluoride. J. f.ür. Prakt. Chem. 117, 1–82 (1927).
Feng, C. Y., Khulbe, K. C., Matsuura, T. & Ismail, A. F. Recent progresses in polymeric hollow fiber membrane preparation, characterization and applications. Sep. Purif. Technol. 111, 43–71 (2013).
Plutschack, M. B., Pieber, B., Gilmore, K. & Seeberger, P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 117, 11796–11893 (2017).
Acknowledgements
This work was financially supported by the Postdoctoral Fellowship Program of CPSF (GZC20232163, H.L.); the National Natural Science Foundation of China (32372597, Z.H.); the National Key Research and Development Program of China (2020YFA0907903, Z.H.); the 111 project of the Education Ministry of China (No. B18053, F.R.); the Beijing Qi Dian Shi Neng Technology Co., Ltd. We would like to express our deepest gratitude to Prof. Karl Barry Sharpless, Prof. John Moses and Prof. Ross Denton for the insightful discussions.
Author information
Authors and Affiliations
Contributions
J.A. and H.L. designed the experiments. J.A. and F.R. directed the project. H.L., M.P., J.L., L.W., H.D., T.Z. and X.Z. (Xiaoxu Zhang) conducted the experiments. H.L., M.P., K.N., M.W., Z.Y., X.Z. (Xiaohe Zhang) and Z.H. contributed to the data analysis and visualization. J.A. and H.L. prepared the paper with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xuefeng Jiang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Peng, M., Li, J. et al. SO2F2 mediated click chemistry enables modular disulfide formation in diverse reaction media. Nat Commun 15, 8325 (2024). https://doi.org/10.1038/s41467-024-52606-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52606-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.