Abstract
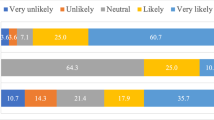
Collagenase Clostridium histolyticum (CCh), the first approved non-surgical treatment for Peyronie’s disease (PD), was withdrawn from the European, Canadian, and Asian markets due to poor demand and lack of government reimbursement options. We sought to assess insurance approval rates and usage of CCh across Canada to understand the factors that led to its withdrawal. Data on patients prescribed CCh for PD or Dupuytren’s contracture was obtained through collaboration with BioScript Solutions to assess the association of variables with insurance approval and prescription filling. We identified 3297 insurance coverage applications for Xiaflex® from April 2018 to June 2020. Of all applications for PD, 92.9% applications were approved while 7.1% were rejected. Despite the withdrawal of CCh from Canadian markets, coverage application approval rates for 2018, 2019, and 2020 were 86.5%, 90.1%, and 89.1%, respectively. Of 2921 approved applications, 88.8% prescriptions were filled. For the 376 rejected applications, 66.4% of prescriptions were filled. Overall, 90% of the cost of Xiaflex® was covered in Canada among those with extended health benefits, with an out-of-pocket expense of $210.4. Insurance coverage requests for Xiaflex® were approved at a high rate in Canada with approved patients being very likely to proceed with therapy, despite interprovincial variation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from BioScript Solutions but restrictions apply to the availability of this data, which was used under license for the current study, and not publicly available. Data are, however, available from the authors upon reasonable request and with permission of BioScript Solutions.
References
Mulhall JP, Creech SD, Boorjian SA, Ghaly S, Kim ED, Moty A, et al. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. J Urol. 2004;171:2350–3.
Gholami SS, Gonzalez-Cadavid NF, Lin CS, Rajfer J, Lue TF. Peyronie’s disease: a review. J Urol. 2003;169:1234–41.
Kadioglu A, Tefekli A, Erol B, Oktar T, Tunc M, Tellaloglu S. A retrospective review of 307 men with Peyronie’s disease. J Urol. 2002;168:1075–9.
Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie’s disease. J Urol. 2006;175:2115–8.
Bella AJ, Lee JC, Grober ED, Carrier S, Benard F, Brock GB. 2018 Canadian Urological Association guideline for Peyronie’s disease and congenital penile curvature. Can Urol Assoc J. 2018;12:E197–209.
Nehra A, Alterowitz R, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh JJ, et al. Peyronie’s disease: AUA guideline. J Urol. 2015;194:745–53.
Gelbard M, Goldstein I, Hellstrom WJG, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207.
Hellstrom WJG, Tue Nguyen HM, Alzweri L, Chung A, Virasoro R, Tapscott A, et al. Intralesional collagenase clostridium histolyticum causes meaningful improvement in men with Peyronie’s disease: results of a multi-institutional analysis. J Urol. 2019;201:777–82.
Paladin Labs Inc. Healthcare Professional Information regarding discontinuation of XIAFLEX®. 2020. https://www.dupuytrencanada.ca/DATA/MEDIA/2022/10/Xiaflex_Discontinuation_Notice_04302020.pdf.
Mann U, Shiff B, Jain K, Flannigan R, Elterman D, Patel P. Canadian provider perspectives on collagenase clostridium histolyticum for the treatment of Peyronie’s disease and the impact of its discontinuation. Int J Impot Res. 2021;34:599–602.
Cordon BH, Hofer MD, Hutchinson RC, Broderick GA, Lotan Y, Morey AF. Superior cost effectiveness of penile plication vs intralesional collagenase injection for treatment of Peyronie’s disease deformities. Urol Pract. 2017;4:118–25.
Harvey N, Pearce I. At what cost is collagenase clostridium histolyticum viable for treating Peyronie’s disease in a public healthcare system? Andrology. 2020;8:1304–11.
Wymer K, Hebert K, Kohler T, Trost LW. MP65-05 Comparative cost-effectiveness of surgery, collagenase clostridium histolyticum, and penile traction therapy in the treatment of Peyronie’s disease. J Urol. 2019;201. https://doi.org/10.1097/01.ju.0000556917.78697.44.
Gamidov S, Shatylko T, Gasanov N, Scherbakov D, Li K, Sukhikh G. Long-term outcomes of surgery for Peyronie’s disease: focus on patient satisfaction. Int J Impot Res. 2021;33:332–8.
Alom M, Sharma KL, Toussi A, Kohler T, Trost L. Efficacy of combined collagenase clostridium histolyticum and RestoreX penile traction therapy in men with Peyronie’s disease. J Sex Med. 2019;16:891–900.
Flores S, Choi J, Alex B, Mulhall JP. Erectile dysfunction after plaque incision and grafting: Short-term assessment of incidence and predictors. J Sex Med. 2011;8:2031–7.
Chung E, Clendinning E, Lessard L, Brock G. Five-year follow-up of Peyronie’s graft surgery: outcomes and patient satisfaction. J Sex Med. 2011;8:594–600.
Punjani N, Nascimento B, Salter C, Miranda E, Terrier J, Taniguchi H, et al. Predictors of depression in men with Peyronie’s disease seeking evaluation. J Sex Med. 2021;18:783–8.
Nelson CJ, Mulhall JP. Psychological impact of Peyronie’s disease: a review. J Sex Med. 2013;10:653–60.
Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie’s disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009;41:467–71.
Russo GI, Milenkovic U, Hellstrom W, Levine LA, Ralph D, Albersen M. Clinical efficacy of injection and mechanical therapy for Peyronie’s disease: a systematic review of the literature. Eur Urol. 2018;74:767–81.
Stuntz M, Perlaky A, Des Vignes F, Kyriakides T, Glass D. The prevalence of Peyronie’s disease in the United States: a population-based study. PLoS One. 2016;11:e0150157.
Author information
Authors and Affiliations
Contributions
DC, BS, DSB, TS, RBB, MG, RF, and PP each contributed to the study design, manuscript drafting, approval of the final version, and agree to be accountable for the accuracy of the work.
Corresponding author
Ethics declarations
Competing interests
RF has received speaking honoraria and an education grant from Boston Scientific and speaking honoraria from Paladin Labs. PP is a consultant for Boston Scientific. The remaining authors have no conflicts of interest to declare.
Ethical approval
Research ethics board approval as per TCPS2 was not required in this study as all data from BioScript Solutions is previously anonymized and no confidential patient information was accessible.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, D., Shiff, B., Bal, D.S. et al. Insurance approval rates for collagenase clostridium histolyticum prior to discontinuation: a Canada-wide analysis. Int J Impot Res (2023). https://doi.org/10.1038/s41443-023-00749-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-023-00749-7