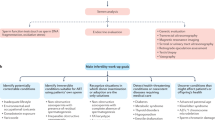
Abstract
Male factor infertility (MFI) is a rising issue worldwide with significant socioeconomic costs and negative psychological consequences for the couple. Current guidelines provide recommendations for its diagnosis and treatment but several gaps in the management of MFI are encountered in clinical practice due to the lack of available evidence in published literature. Uncertainty in the management of MFI cases leads to a high degree of variability in therapeutic approaches. We planned a Delphi consensus method to provide insights and help bridge the gaps that separate clinical guidelines from real-world practice. The Advisory Board collected 41 statements on debated topics in the management of MFI, each including multiple items designed as a 5-point Likert scale. The questionnaire was sent by e-mail to a panel of Italian experts for a first round of voting; members of the panel were later invited to a second round of voting, preceded by discussion of the “hot topics” identified in the first round. At both rounds of the Delphi consensus 68 experts participated to the voting process. After the first round 25 statements were identified as hot topics, and these underwent the second round of voting. Consensus was reached on many, but not all cases, leaving vagueness on few debated topics where decisions are unsupported by clinical studies or driven by controversial results. In conclusion, indications emerging from this large panel of experts may help guide the management of male factor infertility in clinical practice. Studies are needed to address unanswered questions left by cases for whom no consensus was reached.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
04 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41443-022-00543-x
References
World Health Organization. Infertility definitions and terminology. https://www.who.int/reproductivehealth/topics/infertility/definitions/en/. Accessed March 28, 2020. n.d.
Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu JI, et al. EAU Guidelines on Sexual and Reproductive Health. 2021. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Sexual-and-Reproductive-Health-2021-V3.pdf n.d.
Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril. 2021;115:54–61. https://doi.org/10.1016/j.fertnstert.2020.11.015
Pozzi E, Boeri L, Candela L, Capogrosso P, Cazzaniga W, Fallara G, et al. Infertile couples still undergo assisted reproductive treatments without initial andrological evaluation in the real-life setting: A failure to adhere to guidelines? Andrology 2021. https://doi.org/10.1111/andr.13071
Fallara G, Cazzaniga W, Boeri L, Capogrosso P, Candela L, Pozzi E, et al. Male factor infertility trends throughout the last 10 years: report from a tertiary-referral academic andrology centre. Andrology. 2021;9:610–7. https://doi.org/10.1111/andr.12947
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. https://doi.org/10.1093/humupd/dmp048
Campbell SM, Cantrill JA, Roberts D. Prescribing indicators for UK general practice: Delphi consultation study. BMJ. 2000;321:425–8. https://doi.org/10.1136/bmj.321.7258.425
McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–62. https://doi.org/10.1007/s11096-016-0257-x
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE. 2011;6:e20476. https://doi.org/10.1371/journal.pone.0020476
Dalkey N, Brown B, Cochran S. The Delphi Method, III: Use of self ratings to improve group estimates. Santa Monica, CA: RAND Corporation; 1969. n.d.
Boeri L, Capogrosso P, Ventimiglia E, Cazzaniga W, Pozzi E, Belladelli F, et al. Testicular volume in infertile versus fertile white-European men: a case-control investigation in the real-life setting. Asian J Androl. 2021. https://doi.org/10.4103/aja.aja_93_20
Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD007411.pub3
Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, et al. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J Mens Health 2021. https://doi.org/10.5534/wjmh.200196
De Rocco Ponce M, Foresta C, Rago R, Dal Lago A, Balercia G, Calogero AE, et al. Use of biosimilar follicle-stimulating hormone in asthenozoospermic infertile patients: a multicentric study. J Clin Med. 2020;9:E1478. https://doi.org/10.3390/jcm9051478
Monga M, Alexandrescu B, Katz SE, Stein M, Ganiats T. Impact of infertility on quality of life, marital adjustment, and sexual function. Urology. 2004;63:126–30. https://doi.org/10.1016/j.urology.2003.09.015
Wischmann TH. Sexual disorders in infertile couples. J Sex Med. 2010;7:1868–76. https://doi.org/10.1111/j.1743-6109.2010.01717.x
Capogrosso P, Jensen CFS, Rastrelli G, Torremade J, Russo GI, Raheem AA, et al. Male sexual dysfunctions in the infertile couple-recommendations from the European Society of Sexual Medicine (ESSM). Sex Med. 2021;9:100377. https://doi.org/10.1016/j.esxm.2021.100377
Capogrosso P, Ventimiglia E, Boeri L, Cazzaniga W, Chierigo F, Montorsi F, et al. Male infertility as a proxy of the overall male health status. Minerva Urol Nefrol. 2018;70:286–99. https://doi.org/10.23736/S0393-2249.18.03063-1
Pozzi E, Boeri L, Capogrosso P, Candela L, Cazzaniga W, Belladelli F, et al. Infertility as a Proxy of Men’s Health: Still a long way to go. Turk J Urol. 2021. https://doi.org/10.5152/tud.2021.20561
Isidori AM, Giammusso B, Corona G, Verze P. Diagnostic and therapeutic workup of erectile dysfunction: results from a Delphi consensus of andrology experts. Sex Med. 2019;7:292–302. https://doi.org/10.1016/j.esxm.2019.04.001
Collins JW, Ghazi A, Stoyanov D, Hung A, Coleman M, Cecil T, et al. Utilising an accelerated Delphi process to develop guidance and protocols for telepresence applications in remote robotic surgery training. Eur Urol Open Sci. 2020;22:23–33. https://doi.org/10.1016/j.euros.2020.09.005
Boeri L, Belladelli F, Capogrosso P, Cazzaniga W, Candela L, Pozzi E, et al. Normal sperm parameters per se do not reliably account for fertility: a case–control study in the real-life setting. Andrologia. 2021;53:e13861. https://doi.org/10.1111/and.13861
Santi D, Spaggiari G, Simoni M, Sperm DNA. fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management—meta-analyses. Reprod Biomed Online. 2018;37:315–26. https://doi.org/10.1016/j.rbmo.2018.06.023
Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health. 2020;38:412–71. https://doi.org/10.5534/wjmh.200128
Haddock L, Gordon S, Lewis SEM, Larsen P, Shehata A, Shehata H. Sperm DNA fragmentation is a novel biomarker for early pregnancy loss. Reprod Biomed Online. 2021;42:175–84. https://doi.org/10.1016/j.rbmo.2020.09.016
Wang Y-J, Zhang R-Q, Lin Y-J, Zhang R-G, Zhang W-L. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. https://doi.org/10.1016/j.rbmo.2012.05.002
Qiu D, Shi Q, Pan L. Efficacy of varicocelectomy for sperm DNA integrity improvement: a meta-analysis. Andrologia. 2021;53:e13885. https://doi.org/10.1111/and.13885
NICE Clinical Guideline 2013: Fertility: assessment and treatment for people with fertility problems. Accessed August 24th 2021. https://www.nice.org.uk/guidance/cg156/evidence/full-guideline-pdf-188539453
Lähteenmäki A, Räsänen M, Hovatta O. Low-dose prednisolone does not improve the outcome of in-vitro fertilization in male immunological infertility. Hum Reprod. 1995;10:3124–9. https://doi.org/10.1093/oxfordjournals.humrep.a135871
Haas GG, Manganiello P. A double-blind, placebo-controlled study of the use of methylprednisolone in infertile men with sperm-associated immunoglobulins. Fertil Steril. 1987;47:295–301.
Bals-Pratsch M, Dören M, Karbowski B, Schneider HP, Nieschlag E. Cyclic corticosteroid immunosuppression is unsuccessful in the treatment of sperm antibody-related male infertility: a controlled study. Hum Reprod. 1992;7:99–104. https://doi.org/10.1093/oxfordjournals.humrep.a137568
Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for Management of Idiopathic male infertility. World J Mens Health. 2019;37:296–312. https://doi.org/10.5534/wjmh.190055
Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470–85. https://doi.org/10.1038/nrurol.2017.69
Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. https://doi.org/10.1200/JCO.2006.06.5888
Quinn GP, Vadaparampil ST, Lee J-H, Jacobsen PB, Bepler G, Lancaster J, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. https://doi.org/10.1200/JCO.2009.23.0250
Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. https://doi.org/10.1001/archinternmed.2008.562
Boeri L, Ventimiglia E, Cazzaniga W, Pederzoli F, Fallara G, Pozzi E, et al. Risk of health status worsening in primary infertile men: a prospective 10-year follow-up study. Andrology. 2021. https://doi.org/10.1111/andr.13090
Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56–83. https://doi.org/10.1093/humupd/dmu042
Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol. 2015;25:323–30. https://doi.org/10.1007/s00330-014-3437-x
Barbonetti A, Martorella A, Minaldi E, D’Andrea S, Bardhi D, Castellini C, et al. Testicular cancer in infertile men with and without testicular microlithiasis: a systematic review and meta-analysis of case–control studies. Front Endocrinol. 2019;10:164. https://doi.org/10.3389/fendo.2019.00164
Calogero AE, Duca Y, Condorelli RA, La Vignera S. Male accessory gland inflammation, infertility, and sexual dysfunctions: a practical approach to diagnosis and therapy. Andrology. 2017;5:1064–72. https://doi.org/10.1111/andr.12427
Gimenes F, Souza RP, Bento JC, Teixeira JJV, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11:672–87. https://doi.org/10.1038/nrurol.2014.285
Boeri L, Pederzoli F, Capogrosso P, Abbate C, Alfano M, Mancini N, et al. Semen infections in men with primary infertility in the real-life setting. Fertil Steril. 2020;113:1174–82. https://doi.org/10.1016/j.fertnstert.2020.01.034
Fode M, Fusco F, Lipshultz L, Weidner W. Sexually transmitted disease and male infertility: a systematic review. Eur Urol Focus. 2016;2:383–93. https://doi.org/10.1016/j.euf.2016.08.002
Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod. 2019;34:209–17. https://doi.org/10.1093/humrep/dey348
Xiong Y-Q, Chen Y-X, Cheng M-J, He W-Q, Chen Q. The risk of human papillomavirus infection for male fertility abnormality: a meta-analysis. Asian J Androl. 2018;20:493–7. https://doi.org/10.4103/aja.aja_77_17
Depuydt CE, Donders GGG, Verstraete L, Vanden Broeck D, Beert JFA, Salembier G, et al. Infectious human papillomavirus virions in semen reduce clinical pregnancy rates in women undergoing intrauterine insemination. Fertil Steril. 2019;111:1135–44. https://doi.org/10.1016/j.fertnstert.2019.02.002
Rastrelli G, Lotti F, Reisman Y, Sforza A, Maggi M, Corona G. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expert Rev Endocrinol Metab. 2019;14:321–34. https://doi.org/10.1080/17446651.2019.1657827
Ibañez-Perez J, Santos-Zorrozua B, Lopez-Lopez E, Matorras R, Garcia-Orad A. An update on the implication of physical activity on semen quality: a systematic review and meta-analysis. Arch Gynecol Obstet. 2019;299:901–21. https://doi.org/10.1007/s00404-019-05045-8
Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory methods for the examination of human semen. Eur Urol. 2016;70:635–45. https://doi.org/10.1016/j.eururo.2016.04.010
Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl. 2019;21:478–85. https://doi.org/10.4103/aja.aja_110_18
Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Datab Syst Rev. 2019;3:CD007411. https://doi.org/10.1002/14651858.CD007411.pub4
Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, et al. The effect of antioxidants on male factor infertility: the males, antioxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113:552–60. https://doi.org/10.1016/j.fertnstert.2019.11.008
Pyrgidis N, Sokolakis I, Palapelas V, Tishukov M, Mykoniatis I, Symeonidis EN, et al. The effect of antioxidant supplementation on operated or non-operated varicocele-associated infertility: a systematic review and meta-analysis. Antioxidants. 2021;10:1067. https://doi.org/10.3390/antiox10071067
Liu K-S, Mao X-D, Pan F, An RF. Effect and mechanisms of reproductive tract infection on oxidative stress parameters, sperm DNA fragmentation, and semen quality in infertile males. Reprod Biol Endocrinol. 2021;19:97. https://doi.org/10.1186/s12958-021-00781-6
Boeri L, Capogrosso P, Salonia A Gonadotropin treatment for the male hypogonadotropic hypogonadism. Curr Pharm Des. 2020. https://doi.org/10.2174/1381612826666200523175806
Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril. 2006;86:1664–8. https://doi.org/10.1016/j.fertnstert.2006.05.042
Sharma D, Zillioux J, Khourdaji I, Reines K, Wheeler K, Costabile R, et al. Improvements in semen parameters in men treated with clomiphene citrate-A retrospective analysis. Andrologia. 2019;51:e13257. https://doi.org/10.1111/and.13257
Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1:749–57. https://doi.org/10.1111/j.2047-2927.2013.00107.x
Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Calogero AE, La Vignera S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert Opin Pharmacother. 2019;20:1517–25. https://doi.org/10.1080/14656566.2019.1615057
Santi D, Granata ARM, Simoni M. FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis. Endocr Connect. 2015;4:R46–58. https://doi.org/10.1530/EC-15-0050
Cannarella R, La Vignera S, Condorelli RA, Mongioì LM, Calogero AE. FSH dosage effect on conventional sperm parameters: a meta-analysis of randomized controlled studies. Asian J Androl. 2020;22:309–16. https://doi.org/10.4103/aja.aja_42_19
Barbonetti A, Calogero AE, Balercia G, Garolla A, Krausz C, La Vignera S, et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J Endocrinol Invest. 2018;41:1107–22. https://doi.org/10.1007/s40618-018-0843-y
Attia AM, Abou-Setta AM, Al-Inany HG Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD005071.pub4
Simoni M, Santi D, Negri L, Hoffmann I, Muratori M, Baldi E, et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p.N680S: a pharmacogenetic study. Hum Reprod. 2016;31:1960–9. https://doi.org/10.1093/humrep/dew167
Garolla A, Ghezzi M, Cosci I, Sartini B, Bottacin A, Engl B, et al. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine. 2017;56:416–25. https://doi.org/10.1007/s12020-016-1037-z
Ding Y, Zhang X, Li J-P, Chen S, Zhang R, Tan W, et al. Treatment of idiopathic oligozoospermia with recombinant human follicle-stimulating hormone: a prospective, randomized, double-blind, placebo-controlled clinical study in Chinese population. Clin Endocrinol. 2015;83:866–71. https://doi.org/10.1111/cen.12770
Simoni M, Santi D. FSH treatment of male idiopathic infertility: Time for a paradigm change. Andrology. 2020;8:535–44. https://doi.org/10.1111/andr.12746
La Vignera S, Condorelli RA, Duca Y, Mongioi LM, Cannarella R, Giacone F, et al. FSH therapy for idiopathic male infertility: four schemes are better than one. Aging Male. 2020;23:750–5. https://doi.org/10.1080/13685538.2019.1590696
Casamonti E, Vinci S, Serra E, Fino MG, Brilli S, Lotti F, et al. Short-term FSH treatment and sperm maturation: a prospective study in idiopathic infertile men. Andrology. 2017;5:414–22. https://doi.org/10.1111/andr.12333
Foresta C, Bettella A, Ferlin A, Garolla A, Rossato M. Evidence for a stimulatory role of follicle-stimulating hormone on the spermatogonial population in adult males. Fertil Steril. 1998;69:636–42. https://doi.org/10.1016/s0015-0282(98)00008-9
Garolla A, Selice R, Engl B, Bertoldo A, Menegazzo M, Finos L, et al. Spermatid count as a predictor of response to FSH therapy. Reprod Biomed Online. 2014;29:102–12. https://doi.org/10.1016/j.rbmo.2014.02.014
Ni K, Steger K, Yang H, Wang H, Hu K, Zhang T, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–24. https://doi.org/10.1111/andr.12210
Lira Neto FT, Roque M, Esteves SC. Effect of varicocelectomy on sperm deoxyribonucleic acid fragmentation rates in infertile men with clinical varicocele: a systematic review and meta-analysis. Fertil Steril. 2021. https://doi.org/10.1016/j.fertnstert.2021.04.003
Smit M, Romijn JC, Wildhagen MF, Veldhoven JLM, Weber RFA, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189:S146–150. https://doi.org/10.1016/j.juro.2012.11.024
Tharakan T, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. European Association of Urology Guidelines Panel on male sexual and reproductive health: a Clinical Consultation Guide on the indications for performing sperm DNA fragmentation testing in men with infertility and testicular sperm extraction in nonazoospermic men. Eur Urol Focus. 2021. https://doi.org/10.1016/j.euf.2020.12.017
Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl. 2016;18:246–53. https://doi.org/10.4103/1008-682X.169562
Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016;106:1338–43. https://doi.org/10.1016/j.fertnstert.2016.07.1093
Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic review. Andrology. 2014;2:20–4. https://doi.org/10.1111/j.2047-2927.2013.00148.x
Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril. 2015;104:1099–1103.e1-3. https://doi.org/10.1016/j.fertnstert.2015.07.1136
Corona G, Minhas S, Giwercman A, Bettocchi C, Dinkelman-Smit M, Dohle G, et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:733–57. https://doi.org/10.1093/humupd/dmz028
Maglia E, Boeri L, Fontana M, Gallioli A, De Lorenzis E, Palmisano F, et al. Clinical comparison between conventional and microdissection testicular sperm extraction for non-obstructive azoospermia: understanding which treatment works for which patient. Arch Ital Urol Androl. 2018;90:130–5. https://doi.org/10.4081/aiua.2018.2.130
Cocci A, Cito G, Russo GI, Falcone M, Capece M, Timpano M, et al. Effectiveness of highly purified urofollitropin treatment in patients with idiopathic azoospermia before testicular sperm extraction. Urologia. 2018;85:19–21. https://doi.org/10.5301/uj.5000253
Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111:E110–114. https://doi.org/10.1111/j.1464-410X.2012.11485.x
Shiraishi K, Ohmi C, Shimabukuro T, Matsuyama H. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012;27:331–9. https://doi.org/10.1093/humrep/der404
Acknowledgments
Authors want to acknowledge all collaborators who completed the questionnaire during the first and second round of voting: Paolo Turchi, Gianmartin Cito, Ilaria Natali, Alessandro Natali, Antonio Corvasce, Lucilla Divenuto, Stefano Impedovo, Michele Tedeschi, Francesco Paolo Turri, Antonio Vavallo, Antonio Vitarelli, Francesco Sebastiani, Davide Arcaniolo, Francesco Bottone, Francesco Chiancone, Lorenzo Cirigliano, Michelangelo Sorrentino, Giuseppina Peluso, Ottavio Sicuro, Pietro Paolo Cozza, Manuela Andreozzi, Marco Bitelli, Giorgio Franco, Vincenzo Gentile, Giuseppe La Pera, Andrea Ortensi, Pietro Salacone, Federica Sanna, Giovanni Tuffo, Paola Asero, Danilo Di Trapani, Vincenzo Favilla, Ignazio Gattuccio, Emilio Italiano, Bruno Giammusso, Filippo Montalto, Paolo Panella, Salvatore Privitera, Pietro Russo, Giuseppe Sidoti, Andrea Fabiani, Giorgio Gentile, Alessandro Franceschelli, Carlo Maretti, Edoardo Pescatori, Pasquale Scarano, Massimo Polito, Luigi Quaresima, Andrea Salonia, Gaetano Donatelli, Antonio Avolio, Daniele Tiscione, Andrea Galantini, Matteo Titta, Giorgio Piubello, Luca Boeri, Massimo Iafrate, Filippo Migliorni, Giovanni Liguori, Gioacchino De Giorgi, Emanuele Baldassarre, Giorgio Del Noce, Michele Manica, Carla Pasquale, Maurizio Ruggieri, Paolo Capogrosso, Fabrizio Ildefonso Scroppo, Elisabetta Micelli, Michele Rizzo.
Author information
Authors and Affiliations
Contributions
LB was responsible for designing the protocol, collecting data, interpreting results and drafting the manuscript. PC was responsible for designing the protocol, collecting data, interpreting results. IO was responsible for designing the protocol, collecting data, interpreting results. CM was responsible for designing the protocol, collecting data, interpreting results. TC was responsible for designing the protocol, collecting data, interpreting results lts. PV was responsible for designing the protocol, collecting data, interpreting results. AS was responsible for designing the protocol, collecting data, interpreting results. BG was responsible for designing the protocol, collecting data, interpreting results. AP was responsible for designing the protocol, collecting data, interpreting results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add acknowledgments.
Supplementary information
Rights and permissions
About this article
Cite this article
Boeri, L., Capogrosso, P., Ortensi, I. et al. Diagnostic and therapeutic workup of male infertility: results from a Delphi consensus panel. Int J Impot Res 35, 1–13 (2023). https://doi.org/10.1038/s41443-021-00511-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-021-00511-x
This article is cited by
-
Re: Diagnostic and therapeutic workup of male infertility: results from a Delphi Consensus Panel
International Journal of Impotence Research (2023)