Abstract
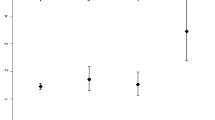
We previously reported that brain atrophy was more severe and progressed more rapidly in patients with end-stage kidney disease on peritoneal dialysis (PD) than those with non-dialysis-dependent chronic kidney disease. However, it remains unknown whether there is a difference between patients on PD and hemodialysis (HD). In total, 73 PD and 34 HD patients who underwent brain magnetic resonance imaging (MRI) were recruited for a cross-sectional analysis. Among them, 42 PD and 25 HD patients who underwent a second brain MRI after 2 years were recruited for a longitudinal analysis. T1-weighted MRI images were analyzed. Total gray matter volume (GMV), total white matter volume, and cerebrospinal fluid volume were segmented, and each volume was quantified using statistical parametric mapping software. The ratio of GMV (GMR) was calculated by dividing GMV by intracranial volume, to adjust for variations in head size. We compared GMR between PD and HD patients in the cross-sectional analysis and the annual change in GMR (AC-GMR) in the longitudinal analysis. In the cross-sectional analysis, age- and sex-adjusted GMR was significantly lower in PD than HD patients [least square mean (LSM): 39.2% vs. 40.0%, P = 0.018]. AC-GMR was significantly greater in PD than HD patients and this difference remained significant even after adjustment for potential confounding factors (LSM: −0.68 vs. −0.28 percentage-points/year, P = 0.011). In conclusion, the present study demonstrated a more rapid progression of brain atrophy in PD patients compared with HD patients.

We demonstrated that decline in GMR progressed significantly more rapidly in PD than HD patients independent of potential confounding factors. GMR gray matter volume ratio, HD hemodialysis, PD peritoneal dialysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tsuruya K, Yoshida H, Haruyama N, Fujisaki K, Hirakata H, Kitazono T. Clinical significance of fronto-temporal gray matter atrophy in executive dysfunction in patients with chronic kidney disease: The VCOHP Study. PLoS One. 2015;10:e0143706.
Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–63.
Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–34.
Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing type 1 diabetes. Diabetes. 2011;60:315–9.
Hayashi K, Kurioka S, Yamaguchi T, Morita M, Kanazawa I, Takase H, et al. Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:180–5.
Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–41.
Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–9.
Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248:590–8.
Jokinen H, Lipsanen J, Schmidt R, Fazekas F, Gouw AA, van der Flier WM, et al. Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology. 2012;78:1785–92.
Zhang LJ, Wen J, Ni L, Zhong J, Liang X, Zheng G, Lu GM. Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis. 2013;28:647–54.
Yoshimitsu T, Hirakata H, Fujii K, Kanai H, Hirakata E, Higashi H, et al. Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin Nephrol. 2000;53:445–51.
Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, et al. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97:c23–30.
Tsuruya K, Yoshida H, Kuroki Y, Nagata M, Mizumasa T, Mitsuiki K, et al. Brain atrophy in peritoneal dialysis and CKD stages 3-5: a cross-sectional and longitudinal study. Am J Kidney Dis. 2015;65:312–21.
Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399–2400.
Yoshida H, Kawaguchi A, Tsuruya K. Radial basis function-sparse partial least squares for application to brain imaging data. Comput Math Methods Med. 2013;2013:591032.
Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging. 2011;32:907–15.
Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6:e22734.
Wolfgram DF, Szabo A, Murray AM, Whittle J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int. 2015;35:189–98.
O'lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67:925–35.
Tian X, Guo X, Xia X, Yu H, Li X, Jiang A. The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14390.
Chen HJ, Qiu J, Qi Y, Fu L, Fu Q, Wu W, et al. Hippocampal subfield morphology in regular hemodialysis patients. Nephrol Dial Transplant. 2023;38:992–1001.
Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61:564–9.
Kanai H, Hirakata H, Nakane H, Fujii K, Hirakata E, Ibayashi S, Kuwabara Y. Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis. 2001;38:S129–S133.
Hirakata H, Yao H, Osato S, Ibayashi S, Onoyama K, Otsuka M, et al. CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Physiol. 1992;262:F737–F743.
Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480–6.
Temple RM, Langan SJ, Deary IJ, Winney RJ. Recombinant erythropoietin improves cognitive function in chronic haemodialysis patients. Nephrol Dial Transplant. 1992;7:240–5.
Park SE, Kim H, Lee J, Lee NK, Hwang JW, Yang JJ, et al. Decreased hemoglobin levels, cerebral small-vessel disease, and cortical atrophy: among cognitively normal elderly women and men. Int Psychogeriatr. 2016;28:147–56.
Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–8.
Kamata T, Hishida A, Takita T, Sawada K, Ikegaya N, Maruyama Y, et al. Morphologic abnormalities in the brain of chronically hemodialyzed patients without cerebrovascular disease. Am J Nephrol. 2000;20:27–31.
USRDS 2023 Annual Report, End Stage Renal Disease: Chapter 11, International Comparison. https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/11-international-comparisons. Accessed 10 November (2023).
Acknowledgements
We greatly appreciate Prof. Takashi Yoshiura (Department of Radiology, Kagoshima University Graduate School of Medical and Dental Sciences) for technical advice on the analysis of the MRI data and the staff of the Advanced Preventive Medical Center in Kyushu University Hospital for their kind cooperation. We also appreciate Drs. Koji Mitsuiki (Harasanshin Hospital), Hideki Hirakata (Fukuoka Renal Clinic), Takashi Nagae (National Hospital Organization Fukuoka-higashi Medical Center), Masatomo Taniguchi (Fukuoka Renal Clinic), Yasuhiro Kawai (Steel Memorial Yawata Hospital), Yasuhisa Tamura (JCHO Kyushu Hospital), Michiya Shinozaki (Shin-Yurigaoka General Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Takashi Deguchi (Hamanomachi Hospital), Dai Matsuo (Munakata Medical Association Hospital), Izumi Shimano (Munakata Medical Association Hospital), Yusuke Kuroki (National Hospital Organization Fukuoka-higashi Medical Center), Itsuko Ishida (Gofukumachi Kidney Clinic, Harasanshin Hospital), Kei Hori (Hori Clinic), Shotaro Ohnaka (Tagawa Municipal Hospital), Hiroshi Tsuruta (Kokura Daiichi Hospital), Toru Mizumasa (Kyushu Central Hospital), Makoto Hirakawa (Hirakawa Internal Medicine Clinic), Takahiro Yoshimitsu (Fukuoka Mirai Hospital), Kiyoshi Ikeda (Ikeda Vascular Access Dialysis and Internal Medicine Clinic), Koichiro Goto (Goto Clinic), Chiaki Miishima (Miishima Internal Medicine Clinic), Kiichiro Ueno (Ueno Hospital), Takashi Ono (Toma Clinic), Toru Sanai (Fukumitsu Clinic), Takashi Ando (Hakozaki Park Internal Medicine Clinic) for their helpful enrollment of patients to this study. We thank Richard Robins, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Funding
This work was not financially supported by any pharmaceutical company or funding agency.
Author information
Authors and Affiliations
Contributions
Study conception and design: KaT, HY; data acquisition: KaT, HY, SY, NH, ST, AT, ME, KF, KuT, TN, KM; data analysis/interpretation: KaT, HY; statistical analysis: KaT, HY; supervision or mentorship: TK. Each author made important intellectual contributions during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KaT takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no comperting interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuruya, K., Yoshida, H., Yamada, S. et al. More rapid progression of brain atrophy in patients on peritoneal dialysis compared with hemodialysis: The VCOHP Study. Hypertens Res 47, 887–897 (2024). https://doi.org/10.1038/s41440-023-01530-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01530-5
Key words
This article is cited by
-
Salt and seasonal variation research in Asia
Hypertension Research (2024)
-
Brain atrophy in patients on peritoneal dialysis treatment
Hypertension Research (2024)