Abstract
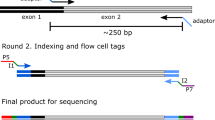
Gene doping, which includes the non-therapeutic use of genes or genetic elements that have the capacity to enhance athletic performance, is prohibited in horseracing and equestrian sports. To provide a comprehensive assessment of matrix and detection techniques, a custom adeno-associated virus serotype 8 vector was designed to include PCR binding sites for multiple target genes and assay types. The vector was injected via an intramuscular route into two Thoroughbred horses and matrices collected at defined timepoints. DNA was analysed using 3 detection methods: qPCR, digital PCR, and NGS. Overall, there was a strong correlation across the different detection methods employed, although digital PCR was less sensitive at lower concentrations. High concentrations of vector were detected at early timepoints in plasma and whole blood, which rapidly dropped after 0.5 d to trace levels by 4 d and 9 d post-administration respectively, following a similar pattern to previous studies. Vector was detected in dried blood spots at lower levels than whole blood, but with a similar detection time. Detection in hair root bulbs in one horse was observed at over a month post-administration, which opens new avenues for future gene doping testing in humans and animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequence information, computer code and resources are available by permission from the British Horseracing Authority under an appropriate materials transfer agreement.
References
Kattenhorn LM, Tipper CH, Stoica L, Geraghty DS, Wright TL, Clark KR, et al. Adeno-Associated Virus Gene Therapy for Liver Disease. Hum Gene Ther. 2016;27:947.
Kovac M, Litvin YA, Aliev RO, Zakirova EY, Rutland CS, Kiyasov AP, et al. Gene Therapy Using Plasmid DNA Encoding VEGF164 and FGF2 Genes: A Novel Treatment of Naturally Occurring Tendinitis and Desmitis in Horses. Front Pharm. 2018;9:978.
Pozzi D, Caracciolo G. Looking Back, Moving Forward: Lipid Nanoparticles as a Promising Frontier in Gene Delivery. ACS Pharm Transl Sci. 2023;6:1561–73.
Prohibited Substances | FEI. 2023. https://inside.fei.org/fei/cleansport/humans/prohibited-list. Accessed 22 Dec 2023.
International Federation of Horseracing Authorities. 2020. https://www.ifhaonline.org/default.asp?section=IABRWTest&area=2#article6b. Accessed 22 Dec 2023.
Naumann N, Paßreiter A, Thomas A, Krug O, Walpurgis K, Thevis M. Analysis of Potential Gene Doping Preparations for Transgenic DNA in the Context of Sports Drug Testing Programs. Int J Mol Sci. 2023;24:15835.
Ni W, Le Guiner C, Gernoux G, Penaud-Budloo M, Moullier P, Snyder RO. Longevity of rAAV vector and plasmid DNA in blood after intramuscular injection in nonhuman primates: implications for gene doping. Gene Ther. 2011;18:709–18.
Cheung HW, Wong K, Lin VYC, Farrington AF, Bond AJ, Wan TSM, et al. Optimization and implementation of four duplex quantitative polymerase chain reaction assays for gene doping control in horseracing. Drug Test Anal. 2022;14:1587–98.
Garton FC, Houweling PJ, Vukcevic D, Meehan LR, Lee FXZ, Lek M, et al. The Effect of ACTN3 Gene Doping on Skeletal Muscle Performance. Am J Hum Genet. 2018;102:845–57.
Tozaki T, Hamilton NA. Control of gene doping in human and horse sports. Gene Ther. 2022;29:107–12.
Li R, Su P, Shi Y, Shi H, Ding S, Su X et al. Gene doping detection in the era of genomics. Drug Test Anal. 2024. https://doi.org/10.1002/dta.3664.
Baoutina A, Coldham T, Bains GS, Emslie KR. Gene doping detection: evaluation of approach for direct detection of gene transfer using erythropoietin as a model system. Gene Ther. 2010;17:1022–32.
Tozaki T, Gamo S, Takasu M, Kikuchi M, Kakoi H, Hirota K, et al. Digital PCR detection of plasmid DNA administered to the skeletal muscle of a microminipig: a model case study for gene doping detection. BMC Res Notes. 2018;11:708.
Tozaki T, Ohnuma A, Kikuchi M, Ishige T, Kakoi H, Hirota K, et al. Microfluidic Quantitative PCR Detection of 12 Transgenes from Horse Plasma for Gene Doping Control. Genes. 2020;11:457.
Jiang Z, Haughan J, Moss KL, Stefanovski D, Ortved KF, Robinson MA. A quantitative PCR screening method for adeno‐associated viral vector 2‐mediated gene doping. Drug Test Anal. 2022;14:963–72.
Cheung HW, Wong KS, Lin VYC, Wan TSM, Ho ENM. A duplex qPCR assay for human erythropoietin (EPO) transgene to control gene doping in horses. Drug Test Anal. 2021;13:113–21.
Maniego J, Pesko B, Hincks P, Taylor P, Stewart G, Proudman C, et al. Direct sequence confirmation of qPCR products for gene doping assay validation in horses. Drug Test Anal. 2022;14:1017–25.
Baoutina A, Bhat S, Li DK, Emslie KR. Towards a robust test to detect gene doping for anabolic enhancement in human athletes. Drug Test Anal. 2023;15:314–23.
de Boer EN, van der Wouden PE, Johansson LF, van Diemen CC, Haisma HJ. A next-generation sequencing method for gene doping detection that distinguishes low levels of plasmid DNA against a background of genomic DNA. Gene Ther. 2019;26:338–46.
Maniego J, Pesko B, Habershon-Butcher J, Huggett J, Taylor P, Scarth J, et al. Screening for gene doping transgenes in horses via the use of massively parallel sequencing. Gene Ther. 2022;29:236–46.
Tozaki T, Ohnuma A, Takasu M, Kikuchi M, Kakoi H, Hirota K, et al. Droplet Digital PCR Detection of the Erythropoietin Transgene from Horse Plasma and Urine for Gene-Doping Control. Genes. 2019;10:243.
Ohnuma A, Tozaki T, Kikuchi M, Ishige T, Kakoi H, Hirota K, et al. Multiplex Detection of Transgenes Using πCode Technology for Gene Doping Control. Anal Chem. 2023;95:10149–54.
Yuen BP-N, Wong K-S, So Y-M, Kwok WH, Cheung HW, Wan TSM, et al. Gene Doping Control Analysis of Human Erythropoietin Transgene in Equine Plasma by PCR-Liquid Chromatography High-Resolution Tandem Mass Spectrometry. Anal Chem. 2024;96:5307–14.
Marchand A, Roulland I, Semence F, Ericsson M. EPO transgene detection in dried blood spots for antidoping application. Drug Test Anal. 2021;13:1888–96.
Laboratory Guidelines - Gene Doping Detection based on Polymerase Chain Reaction (PCR) | World Anti Doping Agency. 2021. https://www.wada-ama.org/en/resources/laboratory-guidelines-gene-doping-detection-based-polymerase-chain-reaction-pcr. Accessed 22 Dec 2023.
Sugasawa T, Aoki K, Yanazawa K, Takekoshi K. Detection of Multiple Transgene Fragments in a Mouse Model of Gene Doping Based on Plasmid Vector Using TaqMan-qPCR Assay. Genes. 2020;11:750.
Haughan J, Jiang Z, Stefanovski D, Moss KL, Ortved KF, Robinson MA. Detection of intra‐articular gene therapy in horses using quantitative real time PCR in synovial fluid and plasma. Drug Test Anal. 2020;12:743–51.
Baoutina A, Bhat S, Zheng M, Partis L, Dobeson M, Alexander IE, et al. Synthetic certified DNA reference material for analysis of human erythropoietin transgene and transcript in gene doping and gene therapy. Gene Ther. 2016;23:708–17.
Tozaki T, Ohnuma A, Kikuchi M, Ishige T, Kakoi H, Hirota KI, et al. Design and storage stability of reference materials for microfluidic quantitative PCR-based equine gene doping tests. J Equine Sci. 2021;32:125.
Prescott MJ, Lidster K. Improving quality of science through better animal welfare: the NC3Rs strategy. Lab Anim. 2017;46:152–6.
Schröder W, Klostermann A, Distl O. Candidate genes for physical performance in the horse. Vet J. 2011;190:39–48.
Ahmetov II, Egorova ES, Gabdrakhmanova LJ, Fedotovskaya ON. Genes and Athletic Performance: An Update. Med Sport Sci. 2016;61:41–54.
Wilkin T, Baoutina A, Hamilton N. Equine performance genes and the future of doping in horseracing. Drug Test Anal. 2017;9:1456–71.
Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Ridwan Amode M, et al. Ensembl 2021. Nucleic Acids Res. 2021;49:D884–91.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115.
Dong JY, Fan PD, Frizzell RA. Quantitative Analysis of the Packaging Capacity of Recombinant Adeno-Associated Virus. Hum Gene Ther. 1996;7:2101–12.
Kalbfleisch TS, Rice ES, DePriest MS, Walenz BP, Hestand MS, Vermeesch JR, et al. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun Biol. 2018;1:197.
Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019;37:761–74.
Ma H, Bell KN, Loker RN. qPCR and qRT-PCR analysis: Regulatory points to consider when conducting biodistribution and vector shedding studies. Mol Ther Methods Clin Dev. 2021;20:152.
la Marca G, Malvagia S, Filippi L, Luceri F, Moneti G, Guerrini R. A new rapid micromethod for the assay of phenobarbital from dried blood spots by LC-tandem mass spectrometry. Epilepsia. 2009;50:2658–62.
Sanmiguel J, Gao G, Vandenberghe LH. Quantitative and Digital Droplet-Based AAV Genome Titration. Methods Mol Biol. 2019;1950:51.
Quail MA, Swerdlow H, Turner DJ, Swerdlow H. Improved protocols for the illumina genome analyzer sequencing system. Curr Protoc Hum Genet. 2009; Chapter 18: Unit 18.2.
Wilkin T, Hamilton NA, Cawley AT, Bhat S, Baoutina A. PCR-Based Equine Gene Doping Test for the Australian Horseracing Industry. Int J Mol Sci. 2024;25:2570.
Wong K, Cheung HW, Szeto CWL, Tsang CYN, Wan TSM, Ho ENM. A multiplex qPCR assay for transgenes detection: A novel approach for gene doping control in horseracing using conventional laboratory setup. Drug Test Anal. 2023;15:879–88.
Whale AS, Huggett JF, Tzonev S. Fundamentals of multiplexing with digital PCR. Biomol Detect Quantif. 2016;10:15–23.
Kumar A, Mhatre S, Godbole S, Jha P, Dikshit R. Optimization of extraction of genomic DNA from archived dried blood spot (DBS): potential application in epidemiological research & bio banking. Gates Open Res. 2019;2:57.
Hengge UR, Mirmohammadsadegh A. Adeno-associated virus expresses transgenes in hair follicles and epidermis. Mol Ther. 2000;2:188–94.
Shen S, Bryant KD, Sun J, Brown SM, Troupes A, Pulicherla N, et al. Glycan Binding Avidity Determines the Systemic Fate of Adeno-Associated Virus Type 9. J Virol. 2012;86:10408.
Vandamme C, Adjali O, Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum Gene Ther. 2017;28:1061.
Acknowledgements
The British Horseracing Authority for funding the analytical work carried out for this study. Staff and students at the Centre for Racehorse Studies (CRS), UK, are also acknowledged for their care and sampling of the horses involved in this work.
Funding
This work was funded by the British Horseracing Authority (BHA).
Author information
Authors and Affiliations
Contributions
ER and PH conceived and secured funding for the study. Experimental data was generated and analysed by JM, CH, and ER. JH-B performed the administration of the rAAV8-CV vector and was responsible for the veterinary health of the animals during the study. All authors were involved in the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Sport and Specialised Analytical Services, LGC Assure, have a commercial interest in developing tests based on gene doping detection.
Ethical approval
Ethical approval was obtained for this study from the BHA Ethics Board. The care and use of all animals in this study were in accordance with the UK Home Office regulations, UK Animals (Scientific Procedures) Act of 1986.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maniego, J., Harding, C., Habershon-Butcher, J. et al. Administration and detection of a multi-target rAAV gene doping vector in horses using multiple matrices and molecular techniques. Gene Ther 31, 477–488 (2024). https://doi.org/10.1038/s41434-024-00462-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-024-00462-0