Abstract
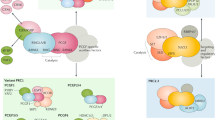
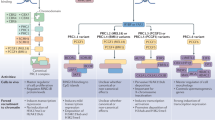
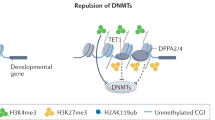
Epigenetic factors are crucial for ensuring proper chromatin dynamics during the initial stages of embryo development. Among these factors, the Polycomb group (PcG) of proteins plays a key role in establishing correct transcriptional programmes during mouse embryogenesis. PcG proteins are classified into two complexes: Polycomb repressive complex 1 (PRC1) and PRC2. Both complexes decorate histone proteins with distinct post-translational modifications (PTMs) that are predictive of a silent transcriptional chromatin state. In recent years, a critical adaptation of the classical techniques to analyse chromatin profiles and to study biochemical interactions at low-input resolution has allowed us to deeply explore PcG molecular mechanisms in the very early stages of mouse embryo development– from fertilisation to gastrulation, and from zygotic genome activation (ZGA) to specific lineages differentiation. These advancements provide a foundation for a deeper understanding of the fundamental role Polycomb complexes play in early development and have elucidated the mechanistic dynamics of PRC1 and PRC2. In this review, we discuss the functions and molecular mechanisms of both PRC1 and PRC2 during early mouse embryo development, integrating new studies with existing knowledge. Furthermore, we highlight the molecular functionality of Polycomb complexes from ZGA through gastrulation, with a particular focus on non-canonical imprinted and bivalent genes, and Hox cluster regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bhakta HH, Refai FH, Avella MA The molecular mechanisms mediating mammalian fertilization. Development. 2019; 146. https://doi.org/10.1242/dev.176966.
Siu KK, Serrão VHB, Ziyyat A, Lee JE The cell biology of fertilization: Gamete attachment and fusion. J Cell Biol. 2021; 220. https://doi.org/10.1083/jcb.202102146.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54.
Burton A, Torres-Padilla M-E. Chromatin dynamics in the regulation of cell fate allocation during early embryogenesis. Nat Rev Mol Cell Biol. 2014;15:723–34.
Xu R, Li C, Liu X, Gao S. Insights into epigenetic patterns in mammalian early embryos. Protein Cell. 2021;12:7–28.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–3.
Schuettengruber B, Bourbon H-M, Di Croce L, Cavalli G. Genome regulation by polycomb and trithorax: 70 years and counting. Cell. 2017;171:34–57.
Piunti A, Shilatifard A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat Rev Mol Cell Biol. 2021;22:326–45.
Aranda S, Mas G, Di Croce L. Regulation of gene transcription by Polycomb proteins. Sci Adv. 2015;1:e1500737.
Chammas P, Mocavini I, Di Croce L. Engaging chromatin: PRC2 structure meets function. Br J Cancer. 2020;122:315–28.
Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–79.
Farcas AM, Blackledge NP, Sudbery I, Long HK, McGouran JF, Rose NR, et al. KDM2B links the polycomb repressive complex 1 (PRC1) to recognition of CpG islands. eLife. 2012;1:e00205.
Zhao J, Wang M, Chang L, Yu J, Song A, Liu C, et al. RYBP/YAF2-PRC1 complexes and histone H1-dependent chromatin compaction mediate propagation of H2AK119ub1 during cell division. Nat Cell Biol. 2020;22:439–52.
Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43.
Gao Z, Zhang J, Bonasio R, Strino F, Sawai A, Parisi F, et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol Cell. 2012;45:344–56.
Højfeldt JW, Hedehus L, Laugesen A, Tatar T, Wiehle L, Helin K. Non-core subunits of the PRC2 complex are collectively required for its target-site specificity. Mol Cell. 2019;76:423–.e3.
Beringer M, Pisano P, Di Carlo V, Blanco E, Chammas P, Vizán P, et al. EPOP functionally links elongin and polycomb in pluripotent stem cells. Mol Cell. 2016;64:645–58.
Conway E, Jerman E, Healy E, Ito S, Holoch D, Oliviero G, et al. A family of vertebrate-specific polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol Cell. 2018;70:408–.e8.
Arecco N, Mocavini I, Blanco E, Ballaré C, Libman E, Bonnal S, et al. Alternative splicing decouples local from global PRC2 activity. Mol Cell. 2024;84:1049–.e8.
Loh CH, Veenstra GJC The role of polycomb proteins in cell lineage commitment and embryonic development. Epigenomes. 2022; 6. https://doi.org/10.3390/epigenomes6030023.
Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–46.
Asenjo HG, Gallardo A, López-Onieva L, Tejada I, Martorell-Marugán J, Carmona-Sáez P, et al. Polycomb regulation is coupled to cell cycle transition in pluripotent stem cells. Sci Adv. 2020;6:eaay4768.
Asenjo HG, Alcazar-Fabra M, Espinosa-Martínez M, Lopez-Onieva L, Gallardo A, Dimitrova E, et al. Changes in PRC1 activity during interphase modulate lineage transition in pluripotent cells. Nat Commun. 2023;14:180.
Tavares L, Dimitrova E, Oxley D, Webster J, Poot R, Demmers J, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148:664–78.
Schoeftner S, Sengupta AK, Kubicek S, Mechtler K, Spahn L, Koseki H, et al. Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. EMBO J. 2006;25:3110–22.
Healy E, Mucha M, Glancy E, Fitzpatrick DJ, Conway E, Neikes HK, et al. PRC2.1 and PRC2.2 synergize to coordinate H3K27 trimethylation. Mol Cell. 2019;76:437–.e6.
Zheng H, Huang B, Zhang B, Xiang Y, Du Z, Xu Q, et al. Resetting epigenetic memory by reprogramming of histone modifications in mammals. Mol Cell. 2016;63:1066–79.
Dahl JA, Jung I, Aanes H, Greggains GD, Manaf A, Lerdrup M, et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature. 2016;537:548–52.
Zhang B, Zheng H, Huang B, Li W, Xiang Y, Peng X, et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature. 2016;537:553–7.
Mei H, Kozuka C, Hayashi R, Kumon M, Koseki H, Inoue A. H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat Genet. 2021;53:539–50.
Chen Z, Djekidel MN, Zhang Y. Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat Genet. 2021;53:551–63.
Zhu Y, Yu J, Rong Y, Wu Y-W, Li Y, Zhang L et al. Genomewide decoupling of H2AK119ub1 and H3K27me3 in early mouse development. Sci Bull. 2021. https://doi.org/10.1016/j.scib.2021.06.010.
Richard Albert J, Greenberg MVC. The Polycomb landscape in mouse development. Nat Genet. 2021;53:427–9.
Liu X, Wang C, Liu W, Li J, Li C, Kou X, et al. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature. 2016;537:558–62.
Du Z, Zheng H, Kawamura YK, Zhang K, Gassler J, Powell S, et al. Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol Cell. 2020;77:825–.e7.
Eckersley-Maslin MA, Alda-Catalinas C, Reik W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat Rev Mol Cell Biol. 2018;19:436–50.
Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–75.
Hanna CW, Kelsey G. Features and mechanisms of canonical and noncanonical genomic imprinting. Genes Dev. 2021;35:821–34.
Chang S, Bartolomei MS. Modeling human epigenetic disorders in mice: Beckwith-Wiedemann syndrome and Silver-Russell syndrome. Dis Model Mech. 2020; 13. https://doi.org/10.1242/dmm.044123.
Inoue A, Jiang L, Lu F, Suzuki T, Zhang Y. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature. 2017;547:419–24.
Inoue A, Chen Z, Yin Q, Zhang Y. Maternal Eed knockout causes loss of H3K27me3 imprinting and random X inactivation in the extraembryonic cells. Genes Dev. 2018;32:1525–36.
Jarred EG, Qu Z, Tsai T, Oberin R, Petautschnig S, Bildsoe H, et al. Transient Polycomb activity represses developmental genes in growing oocytes. Clin Epigenetics. 2022;14:183.
Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961;190:372–3.
Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–7.
Mattimoe T, Payer B. The compleX balancing act of controlling X-chromosome dosage and how it impacts mammalian germline development. Biochem J. 2023;480:521–37.
Boeren J, Gribnau J. Xist-mediated chromatin changes that establish silencing of an entire X chromosome in mammals. Curr Opin Cell Biol. 2021;70:44–50.
Bousard A, Raposo AC, Żylicz JJ, Picard C, Pires VB, Qi Y, et al. The role of Xist-mediated Polycomb recruitment in the initiation of X-chromosome inactivation. EMBO Rep. 2019;20:e48019.
Brockdorff N Polycomb complexes in X chromosome inactivation. Philos Trans R Soc Lond B Biol Sci. 2017; 372. https://doi.org/10.1098/rstb.2017.0021.
Pintacuda G, Wei G, Roustan C, Kirmizitas BA, Solcan N, Cerase A, et al. hnRNPK Recruits PCGF3/5-PRC1 to the Xist RNA B-repeat to establish polycomb-mediated chromosomal silencing. Mol Cell. 2017;68:955–.e10.
Chu C, Zhang QC, da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404–16.
Almeida M, Pintacuda G, Masui O, Koseki Y, Gdula M, Cerase A, et al. PCGF3/5-PRC1 initiates Polycomb recruitment in X chromosome inactivation. Science. 2017;356:1081–4.
Nesterova TB, Wei G, Coker H, Pintacuda G, Bowness JS, Zhang T, et al. Systematic allelic analysis defines the interplay of key pathways in X chromosome inactivation. Nat Commun. 2019;10:3129.
Masui O, Corbel C, Nagao K, Endo TA, Kezuka F, Diabangouaya P, et al. Polycomb repressive complexes 1 and 2 are each essential for maintenance of X inactivation in extra-embryonic lineages. Nat Cell Biol. 2023;25:134–44.
Leung CY, Zernicka-Goetz M. Mapping the journey from totipotency to lineage specification in the mouse embryo. Curr Opin Genet Dev. 2015;34:71–76.
Frum T, Ralston A. Cell signaling and transcription factors regulating cell fate during formation of the mouse blastocyst. Trends Genet. 2015;31:402–10.
Saha B, Home P, Ray S, Larson M, Paul A, Rajendran G, et al. EED and KDM6B coordinate the first mammalian cell lineage commitment to ensure embryo implantation. Mol Cell Biol. 2013;33:2691–705.
Illingworth RS, Hölzenspies JJ, Roske FV, Bickmore WA, Brickman JM. Polycomb enables primitive endoderm lineage priming in embryonic stem cells. eLife. 2016; 5. https://doi.org/10.7554/eLife.14926.
Mohammed H, Hernando-Herraez I, Savino A, Scialdone A, Macaulay I, Mulas C, et al. Single-cell landscape of transcriptional heterogeneity and cell fate decisions during mouse early gastrulation. Cell Rep. 2017;20:1215–28.
Xiang Y, Zhang Y, Xu Q, Zhou C, Liu B, Du Z, et al. Epigenomic analysis of gastrulation identifies a unique chromatin state for primed pluripotency. Nat Genet. 2020;52:95–105.
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26.
Fouse SD, Shen Y, Pellegrini M, Cole S, Meissner A, Van Neste L, et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell. 2008;2:160–9.
Zhang J, Zhang Y, You Q, Huang C, Zhang T, Wang M, et al. Highly enriched BEND3 prevents the premature activation of bivalent genes during differentiation. Science. 2022;375:1053–8.
Mas G, Blanco E, Ballaré C, Sansó M, Spill YG, Hu D, et al. Promoter bivalency favors an open chromatin architecture in embryonic stem cells. Nat Genet. 2018;50:1452–62.
Macrae TA, Fothergill-Robinson J, Ramalho-Santos M. Regulation, functions and transmission of bivalent chromatin during mammalian development. Nat Rev Mol Cell Biol. 2023;24:6–26.
Blanco E, González-Ramírez M, Alcaine-Colet A, Aranda S, Di Croce L. The bivalent genome: characterization, structure, and regulation. Trends Genet. 2020;36:118–31.
Rivera-Pérez JA, Hadjantonakis A-K The dynamics of morphogenesis in the early mouse embryo. Cold Spring Harb Perspect Biol. 2014; 7. https://doi.org/10.1101/cshperspect.a015867.
Argelaguet R, Clark SJ, Mohammed H, Stapel LC, Krueger C, Kapourani C-A, et al. Multi-omics profiling of mouse gastrulation at single-cell resolution. Nature. 2019;576:487–91.
Faust C, Schumacher A, Holdener B, Magnuson T. The eed mutation disrupts anterior mesoderm production in mice. Development. 1995;121:273–85.
Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–71.
O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–6.
Voncken JW, Roelen BAJ, Roefs M, de Vries S, Verhoeven E, Marino S, et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci USA. 2003;100:2468–73.
Illingworth RS, Moffat M, Mann AR, Read D, Hunter CJ, Pradeepa MM, et al. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 2015;29:1897–902.
Blackledge NP, Fursova NA, Kelley JR, Huseyin MK, Feldmann A, Klose RJ. PRC1 catalytic activity is central to polycomb system function. Mol Cell. 2020;77:857–.e9.
Tamburri S, Lavarone E, Fernández-Pérez D, Conway E, Zanotti M, Manganaro D, et al. Histone H2AK119 mono-ubiquitination is essential for polycomb-mediated transcriptional repression. Mol Cell. 2020;77:840–.e5.
Boyle S, Flyamer IM, Williamson I, Sengupta D, Bickmore WA, Illingworth RS. A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes Dev. 2020;34:931–49.
Fursova NA, Blackledge NP, Nakayama M, Ito S, Koseki Y, Farcas AM, et al. Synergy between variant PRC1 complexes defines polycomb-mediated gene repression. Mol Cell. 2019;74:1020–.e8.
Grosswendt S, Kretzmer H, Smith ZD, Kumar AS, Hetzel S, Wittler L, et al. Epigenetic regulator function through mouse gastrulation. Nature. 2020;584:102–8.
Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc. 2006;1:2082–7.
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9.
Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5.
Wang X, Xiang Y, Yu Y, Wang R, Zhang Y, Xu Q, et al. Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Res. 2021;31:526–41.
Sankar A, Mohammad F, Sundaramurthy AK, Wang H, Lerdrup M, Tatar T, et al. Histone editing elucidates the functional roles of H3K27 methylation and acetylation in mammals. Nat Genet. 2022;54:754–60.
Glancy E, Wang C, Tuck E, Healy E, Amato S, Neikes HK, et al. PRC2.1- and PRC2.2-specific accessory proteins drive recruitment of different forms of canonical PRC1. Mol Cell. 2023;83:1393–.e7.
Mochizuki K, Sharif J, Shirane K, Uranishi K, Bogutz AB, Janssen SM, et al. Repression of germline genes by PRC1.6 and SETDB1 in the early embryo precedes DNA methylation-mediated silencing. Nat Commun. 2021;12:7020.
Rothberg JLM, Maganti HB, Jrade H, Porter CJ, Palidwor GA, Cafariello C, et al. Mtf2-PRC2 control of canonical Wnt signaling is required for definitive erythropoiesis. Cell Discov. 2018;4:21.
Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, Motoyama J, et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9:1211–22.
Takeuchi T, Kojima M, Nakajima K, Kondo S. jumonji gene is essential for the neurulation and cardiac development of mouse embryos with a C3H/He background. Mech Dev. 1999;86:29–38.
Højfeldt JW, Laugesen A, Willumsen BM, Damhofer H, Hedehus L, Tvardovskiy A, et al. Accurate H3K27 methylation can be established de novo by SUZ12-directed PRC2. Nat Struct Mol Biol. 2018;25:225–32.
Oksuz O, Narendra V, Lee C-H, Descostes N, LeRoy G, Raviram R, et al. Capturing the onset of PRC2-mediated repressive domain formation. Mol Cell. 2018;70:1149–.e5.
Dobrinić P, Szczurek AT, Klose RJ. PRC1 drives Polycomb-mediated gene repression by controlling transcription initiation and burst frequency. Nat Struct Mol Biol. 2021;28:811–24.
Holoch D, Wassef M, Lövkvist C, Zielinski D, Aflaki S, Lombard B, et al. A cis-acting mechanism mediates transcriptional memory at Polycomb target genes in mammals. Nat Genet. 2021;53:1686–97.
Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LLP, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–59.
Petracovici A, Bonasio R. Distinct PRC2 subunits regulate maintenance and establishment of Polycomb repression during differentiation. Mol Cell. 2021;81:2625–.e5.
Loh CH, van Genesen S, Perino M, Bark MR, Veenstra GJC. Loss of PRC2 subunits primes lineage choice during exit of pluripotency. Nat Commun. 2021;12:6985.
Santanach A, Blanco E, Jiang H, Molloy KR, Sansó M, LaCava J, et al. The Polycomb group protein CBX6 is an essential regulator of embryonic stem cell identity. Nat Commun. 2017;8:1235.
Morey L, Aloia L, Cozzuto L, Benitah SA, Di Croce L. RYBP and Cbx7 define specific biological functions of polycomb complexes in mouse embryonic stem cells. Cell Rep. 2013;3:60–69.
Morey L, Pascual G, Cozzuto L, Roma G, Wutz A, Benitah SA, et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell. 2012;10:47–62.
Morey L, Santanach A, Blanco E, Aloia L, Nora EP, Bruneau BG, et al. Polycomb regulates mesoderm cell fate-specification in embryonic stem cells through activation and repression mechanisms. Cell Stem Cell. 2015;17:300–15.
Leeb M, Pasini D, Novatchkova M, Jaritz M, Helin K, Wutz A. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24:265–76.
Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–46.
Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–19.
Neijts R, Amin S, van Rooijen C, Tan S, Creyghton MP, de Laat W, et al. Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos. Genes Dev. 2016;30:1937–42.
Gaunt SJ, George M, Paul Y-L. Direct activation of a mouse Hoxd11 axial expression enhancer by Gdf11/Smad signalling. Dev Biol. 2013;383:52–60.
Jurberg AD, Aires R, Varela-Lasheras I, Nóvoa A, Mallo M. Switching axial progenitors from producing trunk to tail tissues in vertebrate embryos. Dev Cell. 2013;25:451–62.
Amin S, Neijts R, Simmini S, van Rooijen C, Tan SC, Kester L, et al. Cdx and T Brachyury co-activate growth signaling in the embryonic axial progenitor niche. Cell Rep. 2016;17:3165–77.
Young T, Rowland JE, van de Ven C, Bialecka M, Novoa A, Carapuco M, et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell. 2009;17:516–26.
Durston A, Wacker S, Bardine N, Jansen H. Time space translation: a hox mechanism for vertebrate a-p patterning. Curr Genomics. 2012;13:300–7.
van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–69.
Akasaka T, Kanno M, Balling R, Mieza MA, Taniguchi M, Koseki H. A role for mel-18, a Polycomb group-related vertebrate gene, during theanteroposterior specification of the axial skeleton. Development. 1996;122:1513–22.
Coré N, Bel S, Gaunt SJ, Aurrand-Lions M, Pearce J, Fisher A, et al. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development. 1997;124:721–9.
Takihara Y, Tomotsune D, Shirai M, Katoh-Fukui Y, Nishii K, Motaleb MA, et al. Targeted disruption of the mouse homologue of the Drosophila polyhomeotic gene leads to altered anteroposterior patterning and neural crest defects. Development. 1997;124:3673–82.
Isono K-I, Fujimura Y-I, Shinga J, Yamaki M, O-Wang J, Takihara Y, et al. Mammalian polyhomeotic homologues Phc2 and Phc1 act in synergy to mediate polycomb repression of Hox genes. Mol Cell Biol. 2005;25:6694–706.
Wang S, He F, Xiong W, Gu S, Liu H, Zhang T, et al. Polycomblike-2-deficient mice exhibit normal left-right asymmetry. Dev Dyn. 2007;236:853–61.
Vizán P, Gutiérrez A, Espejo I, García-Montolio M, Lange M, Carretero A, et al. The Polycomb-associated factor PHF19 controls hematopoietic stem cell state and differentiation. Sci Adv. 2020;6:eabb2745.
Lau MS, Schwartz MG, Kundu S, Savol AJ, Wang PI, Marr SK, et al. Mutation of a nucleosome compaction region disrupts Polycomb-mediated axial patterning. Science. 2017;355:1081–4.
Storre J, Elsässer H-P, Fuchs M, Ullmann D, Livingston DM, Gaubatz S. Homeotic transformations of the axial skeleton that accompany a targeted deletion of E2f6. EMBO Rep. 2002;3:695–700.
Huseyin MK, Klose RJ. Live-cell single particle tracking of PRC1 reveals a highly dynamic system with low target site occupancy. Nat Commun. 2021;12:887.
Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science. 2009;324:1320–3.
Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–5.
Noordermeer D, Leleu M, Schorderet P, Joye E, Chabaud F, Duboule D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife. 2014;3:e02557.
Rekaik H, Lopez-Delisle L, Hintermann A, Mascrez B, Bochaton C, Mayran A, et al. Sequential and directional insulation by conserved CTCF sites underlies the Hox timer in stembryos. Nat Genet. 2023;55:1164–75.
Benetti N, Gouil Q, Tapia Del Fierro A, Beck T, Breslin K, Keniry A, et al. Maternal SMCHD1 regulates Hox gene expression and patterning in the mouse embryo. Nat Commun. 2022;13:4295.
Veenvliet JV, Bolondi A, Kretzmer H, Haut L, Scholze-Wittler M, Schifferl D et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science. 2020; 370. https://doi.org/10.1126/science.aba4937.
Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy A-C, et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature. 2018;562:272–6.
Lewis PH. [New mutants report]. Drosoph Inf Serv. 1947;21:69–69.
Jürgens G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature. 1985;316:153–5.
Lewis EB. Genes and developmental pathways. Am Zool. 1963;3:33–56.
Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–70.
Struhl G. A gene product required for correct initiation of segmental determination in Drosophila. Nature. 1981;293:36–41.
Zink B, Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989;337:468–71.
Gaunt SJ, Sharpe PT, Duboule D. Spatially restricted domains of homeo-gene transcripts in mouse embryos: Relation to a segmented body plan. Development. 1988;104:169–79.
Casares F, Sánchez-Herrero E. Regulation of the infraabdominal regions of the bithorax complex of Drosophila by gap genes. Development. 1995;121:1855–66.
Denell RE, Frederick RD. Homoeosis in Drosophila: A description of the polycomb lethal syndrome. Dev Biol. 1983;97:34–47.
Coleman RT, Struhl G Causal role for inheritance of H3K27me3 in maintaining the OFF state of a Drosophila HOX gene. Science. 2017; 356. https://doi.org/10.1126/science.aai8236.
Acknowledgements
We thank Pedro Vizán Carralcázar and Pau Pascual-Garcia for critical reading of the manuscript and insightful discussions, and V.A. Raker for scientific editing. We apologize to the authors whose work we could not discuss due to space limitations. We acknowledge funding from the Spanish Ministry of Economy, Industry and Competitiveness (MEIC) (PID2022-142679NB-I00 and ‘Planes complementarios’ STOP-DMG N6958), “Fundación Vencer El Cancer” (VEC), the European Regional Development Fund (FEDER), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 713673 “ChromDesign”, “la Caixa” Foundation (HR20-00411), and from AGAUR to L.D.C. The Ramon y Cajal programme of the Ministerio de Ciencia, Innovación y Universidades and the European Social Fund under the reference number RYC-2018-025002-I, the Instituto de Salud Carlos III-FEDER (PI22/01837), and MICIU/AEI /10.13039/501100011033 and NextGeneration EU/PRTR (CNS2023-145726) to S.A. We acknowledge the funding support of the Spanish Ministry of Science and Innovation to the EMBL partnership, the Centro de Excelencia Severo Ochoa and the CERCA Programme / Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
LC wrote the manuscript and designed figures. IM wrote and revised the manuscript, and designed figures. SA and LDC conceptualized, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Condemi, L., Mocavini, I., Aranda, S. et al. Polycomb function in early mouse development. Cell Death Differ (2024). https://doi.org/10.1038/s41418-024-01340-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-024-01340-3