Abstract
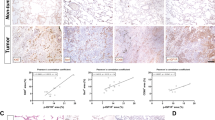
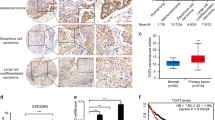
Epidermal growth factor receptor (EGFR) is widely accepted in cancer diagnosis and targeted therapy. Shkbp1 is an upstream molecule of EGFR, which prevents EGFR degradation. However, the role of Shkbp1 in tumor remains to be clarified. Herein we induced tumor in the lungs of Shkbp1 knockout mice with chemical drugs to investigate the function of Shkbp1. Compared with wild-type mice, tumors in the lungs were significantly fewer in Shkbp1 knockout mice. To further explore the biological characteristics and functions of Shkbp1 in cancer cells, we established cell lines with overexpression and low expression of Shkbp1, respectively. Results from our experiments showed that low expression of Shkbp1 in lung cancer remarkably inhibited cancer cell migration and invasion, while overexpression of Shkbp1 promoted their migration and invasion, which indicated that Shkbp1 was closely related with tumor migration and invasion. The mRNA expression analysis of 494 matched tumor and adjacent non-tumor tissues (data derived from TCGA database) revealed that Shkbp1 was associated with the clinic TNM staging. Furthermore, immunohistochemistry (IHC) analysis of tissue microarrays showed that Shkbp1 was also correlated with lymphatic metastasis. Mechanistically, we observed that Shkbp1 was associated with epithelial–mesenchymal transition (EMT) marker. More interestingly, Shkbp1 was also expressed in a variety of immune cells, and we hereby used a subcutaneous transplantation tumor model and a metastasis model created by tail vein injection to explore whether Shkbp1 could impact tumor growth. The results showed that Shkbp1 knockout reduced tumor growth in both tumor models. In general, our results suggest that knocking out Shkbp1 in either immune cells or tumor cells could suppress tumor growth and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Knight SB, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7:170070.
Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature. 2015;526:263–7.
Kosaka T, Tanizaki J, Paranal RM, Endoh H, Lydon C, Capelletti M, et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 2017;77:2712–21.
Day KC, Lorenzatti Hiles G, Kozminsky M, Dawsey SJ, Paul A, Broses LJ, et al. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 2017;77:74–85.
Ng TL, Camidge DR. Lung cancer’s real adjuvant EGFR targeted therapy questions. Lancet Oncol. 2018;19:15–17.
Jiang T, Cheng R, Zhang G, Su C, Zhao C, Li X, et al. Characterization of liver metastasis and its effect on targeted therapy in EGFR-mutant NSCLC: a multicenter study. Clin Lung Cancer. 2017;18:631–9.
Jiang T, Qiao M, Zhou F, Ren S, Su C, Zhou C. Effect of combined therapy inhibiting EGFR and VEGFR pathways in non-small-cell lung cancer on progression-free and overall survival. Clin Lung Cancer. 2017;18:421–31.
Borinstein SC, Hyatt MA, Sykes VW, Straub RE, Lipkowitz S, Boulter J, et al. SETA is a multifunctional adapter protein with three SH3 domains that binds Grb2, Cbl, and the novel SB1 proteins. Cell Signal. 2000;12:769–79.
Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529:110–5.
Feng L, Wang JT, Jin H, Qian K, Geng JG. SH3KBP1-binding protein 1 prevents epidermal growth factor receptor degradation by the interruption of c-Cbl-CIN85 complex. Cell Biochem Funct. 2011;29:589–96.
Liu JP, Liu NS, Yuan HY, Guo Q, Lu H, Li YY. Human homologue of SETA binding protein 1 interacts with cathepsin B and participates in TNF-induced apoptosis in ovarian cancer cells. Mol Cell Biochem. 2006;292:189–95.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95.
Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74.
Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74:309–19.
Singh M, Jadhav HR. Targeting non-small cell lung cancer with small-molecule EGFR tyrosine kinase inhibitors. Drug Discov Today. 2017;23:745–53.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507.
Voon DC, Wang H, Koo JK, Chai JH, Hor YT, Tan TZ, et al. EMT-induced stemness and tumorigenicity are fueled by the EGFR/Ras pathway. PLoS ONE. 2013;8:e70427.
Misra A, Pandey C, Sze SK, Thanabalu T. Hypoxia activated EGFR signaling induces epithelial to mesenchymal transition (EMT). PLoS ONE. 2012;7:e49766.
Nemoto T, Shibata Y, Inoue S, Igarashi A, Tokairin Y, Yamauchi K, et al. MafB silencing in macrophages does not influence the initiation and growth of lung cancer induced by urethane. EXCLI J. 2017;16:914–20.
França WC, Souza AC, Cordeiro JA, Cury PM. Analysis of the action of Himatanthus drasticus in progression of urethane-induced lung cancer in mice. Einstein. 2011;9:350–3.
Matsuyama M, Suzuki H. Strain differences in carcinogenesis by urethane administration to suckling mice with special reference to induction of lung cancer. Br J Cancer. 1968;22:527–32.
Brown M, Farquhar-Smith P. Pain in cancer survivors; filling in the gaps. Br J Anaesth. 2017;119:723–36.
Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–307.
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE. 2009;4:e6816.
Acknowledgements
The authors wish to thank Professor Zhao (Cancer Center of Guangzhou Medical University) for providing valuable help in inducing lung adenocarcinoma in mice with urethane.
Grant support
This work was supported by National Natural Science Foundation of China (Grant ID 81773118 and 31771578) and Key-Area Research and development Program of Guangdong Province (2019B020234003).
Author information
Authors and Affiliations
Contributions
Conception and design: JL and LW. Acquisition of data (the experiments of animal and cell lines): QL, MY, HL, YM, and ZZ. Analysis and interpretation, manuscript revision: QL, HL, XZ, JL, TN, LW. Managed transgenic mice: QL, HL, XH. Statistical analysis, statistical, computation: JL, QL, YM.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Q., Li, H., Yang, M. et al. Suppression of tumor growth and metastasis in Shkbp1 knockout mice. Cancer Gene Ther 29, 709–721 (2022). https://doi.org/10.1038/s41417-021-00349-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00349-x