Abstract
Background
Mesothelioma (MESO) is an insidious malignancy with a complex diagnosis and a poor prognosis. Our study unveils Glutamine-Fructose-6-Phosphate Transaminase 2 (GFPT2) as a valuable diagnostic and prognostic marker for MESO, exploring its role in MESO pathogenesis.
Methods
We utilised tissue samples and clinicopathologic data to evaluate the diagnostic and prognostic significance of GFPT2 as a biomarker for MESO. The role of GFPT2 in the malignant progression of MESO was investigated through in vitro and in vivo experiments. The activation of NF-κB-p65 through O-GlcNAcylation at Ser75 was elucidated using experiments like HPLC-QTRAP-MS/MS and mass spectrometry analysis.
Results
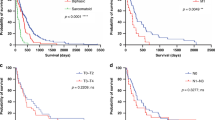
The study demonstrates that GFPT2 exhibits a sensitivity of 92.60% in diagnosing MESO. Overexpression of it has been linked to an unfavourable prognosis. Through rigorous verification, we have confirmed that elevated GFPT2 levels drive malignant proliferation, invasiveness, and metastasis in MESO. At the molecular level, GFPT2 augments p65 O-GlcNAcylation, orchestrating its nuclear translocation and activating the NF-κB signalling pathway.
Conclusions
Our insights suggest GFPT2’s potential as a distinctive biomarker for MESO diagnosis and prognosis, and as an innovative therapeutic target, offering a new horizon for identification and treatment strategies in MESO management.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Yang H, Xu D, Schmid RA, Peng R-W. Biomarker-guided targeted and immunotherapies in malignant pleural mesothelioma. Ther Adv Med Oncol. 2020;12:175883592097142.
Sauter JL, Dacic S, Galateau-Salle F, Attanoos RL, Butnor KJ, Churg A, et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J Thorac Oncol. 2022;17:608–22.
Van Kooten JP, Belderbos RA, Von Der Thüsen JH, Aarts MJ, Verhoef C, Burgers JA, et al. Incidence, treatment and survival of malignant pleural and peritoneal mesothelioma: a population-based study. Thorax. 2022;77:1260–7.
Sinn K, Mosleh B, Hoda MA. Malignant pleural mesothelioma: recent developments. Curr Opin Oncol. 2021;33:80–6.
Miyake N, Ochi N, Yamane H, Fukazawa T, Ikeda T, Yokota E, et al. Targeting ROR1 in combination with pemetrexed in malignant mesothelioma cells. Lung Cancer. 2020;139:170–8.
Bibby AC, Tsim S, Kanellakis N, Ball H, Talbot DC, Blyth KG, et al. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25:472–86.
Monaco SE, Shuai Y, Bansal M, Krasinskas AM, Dacic S. The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol. 2011;135:619–27.
Yoshimura M, Kinoshita Y, Hamasaki M, Matsumoto S, Hida T, Oda Y, et al. Highly expressed EZH2 in combination with BAP1 and MTAP loss, as detected by immunohistochemistry, is useful for differentiating malignant pleural mesothelioma from reactive mesothelial hyperplasia. Lung Cancer. 2019;130:187–93.
Hwang HC, Pyott S, Rodriguez S, Cindric A, Carr A, Michelsen C, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol. 2016;40:714–8.
Shinozaki‐Ushiku A, Ushiku T, Morita S, Anraku M, Nakajima J, Fukayama M. Diagnostic utility of BAP 1 and EZH 2 expression in malignant mesothelioma. Histopathology. 2017;70:722–33.
Hakim SA, Abou Gabal HH. Diagnostic utility of BAP1, EZH2 and Survivin in differentiating pleural epithelioid mesothelioma and reactive mesothelial Hyperplasia: immunohistochemical study. Pathol Oncol Res. 2021;27:600073.
Tolwani A, Matusiak M, Bui N, Forgó E, Varma S, Baratto L, et al. Prognostic relevance of the hexosamine biosynthesis pathway activation in leiomyosarcoma. npj Genom Med. 2021;6:30.
Oki T, Yamazaki K, Kuromitsu J, Okada M, Tanaka I. cDNA cloning and mapping of a novel subtype of Glutamine:fructose-6-phosphate Amidotransferase (GFAT2) in human and mouse. Genomics. 1999;57:227–34.
Szymura SJ, Zaemes JP, Allison DF, Clift SH, D’Innocenzi JM, Gray LG, et al. NF-κB upregulates glutamine-fructose-6-phosphate transaminase 2 to promote migration in non-small cell lung cancer. Cell Commun Signal. 2019;17:24.
Zhang W, Bouchard G, Yu A, Shafiq M, Jamali M, Shrager JB, et al. GFPT2 -Expressing cancer-associated fibroblasts mediate metabolic reprogramming in human lung Adenocarcinoma. Cancer Res. 2018;78:3445–57.
Liu L, Pan Y, Ren X, Zeng Z, Sun J, Zhou K, et al. GFPT2 promotes metastasis and forms a positive feedback loop with p65 in colorectal cancer. Am J Cancer Res. 2020;10:2510–22.
Wang Q, Karvelsson ST, Kotronoulas A, Gudjonsson T, Halldorsson S, Rolfsson O. Glutamine-Fructose-6-Phosphate Transaminase 2 (GFPT2) is upregulated in breast epithelial–mesenchymal transition and responds to oxidative stress. Mol Cell Proteom. 2022;21:100185.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308.
Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20:208–13.
Chiaradonna F, Ricciardiello F, Palorini R. The nutrient-sensing hexosamine biosynthetic pathway as the hub of cancer metabolic rewiring. Cells. 2018;7:53.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNacylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58.
Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–30.
Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–92.
Parker MP, Peterson KR, Slawson C. O-GlcNAcylation and O-GlcNAc cycling regulate gene transcription: emerging roles in cancer. Cancers. 2021;13:1666.
Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820–31.
The UniProt Consortium, Bateman A, Martin M-J, Orchard S, Magrane M, Ahmad S, et al. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523–31.
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res. 2023;51:D1373–80.
Pierce BG, Wiehe K, Hwang H, Kim B-H, Vreven T, Weng Z. ZDOCK server: interactive docking prediction of protein–protein complexes and symmetric multimers. Bioinformatics. 2014;30:1771–3.
Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–97.
Kassambara A, Kosinski M, Biecek P, Fabian Sjdsc. Package ‘survminer.’ Drawing Survival Curves using ‘ggplot2’(R package version 03 1). (2017);
Therneau TM, Lumley T. Package ‘survival.’. R Top Doc 2015;128:28–33.
Suraokar MB, Nunez MI, Diao L, Chow CW, Kim D, Behrens C, et al. Expression profiling stratifies mesothelioma tumors and signifies deregulation of spindle checkpoint pathway and microtubule network with therapeutic implications. Ann Oncol. 2014;25:1184–92.
Knelson EH, Ivanova EV, Tarannum M, Campisi M, Lizotte PH, Booker MA, et al. Activation of tumor-cell STING Primes NK-cell therapy. Cancer Immunol Res. 2022;10:947–61.
Yang F, Li S, Cheng Y, Li J, Han X. Karyopherin α 2 promotes proliferation, migration and invasion through activating NF-κB/p65 signaling pathways in melanoma cells. Life Sci. 2020;252:117611.
Zhou L, Luo M, Cheng L, Li R, Liu B, Linghu H. Glutamine-fructose-6-phosphate transaminase 2 (GFPT2) promotes the EMT of serous ovarian cancer by activating the hexosamine biosynthetic pathway to increase the nuclear location of β-catenin. Pathol - Res Pract. 2019;215:152681.
Kroef V, Ruegenberg S, Horn M, Allmeroth K, Ebert L, Bozkus S, et al. GFPT2/GFAT2 and AMDHD2 act in tandem to control the hexosamine pathway. eLife. 2022;11:e69223.
Stinchcombe TE. Flashback Foreword: Pemetrexed and Cisplatin in Mesothelioma. J Clin Oncol. 2023;41:2123–4.
Carbone M, Adusumilli PS, Alexander HR, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA A Cancer J Clin. 2019;69:402–29.
Yuce TH, Ak G, Metintas S, Dundar E, Roe OD, Panou V, et al. BAP1, Wilms’ tumor 1, and calretinin in predicting survival and response to first-line chemotherapy in patients with pleural mesothelioma. J Cancer Res Clin Oncol. 2024;150:38.
Mohammad T, Garratt J, Torlakovic E, Gilks B, Churg A. Utility of a CEA, CD15, Calretinin, and CK5/6 panel for distinguishing between mesotheliomas and pulmonary adenocarcinomas in clinical practice. Am J Surgical Pathol. 2012;36:1503–8.
Powell G, Roche H, Roche WR. Expression of calretinin by breast carcinoma and the potential for misdiagnosis of mesothelioma. Histopathology. 2011;59:950–6.
Gulyás M, Hjerpe A. Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol. 2003;199:479–87.
Ma G-Y, Shi S, Wang P, Wang X-G, Zhang Z-G. Clinical significance of 9P21 gene combined with BAP1 and MTAP protein expression in diagnosis and prognosis of mesothelioma serous effusion. Biomed Rep. 2022;17:66.
Hung YP, Chirieac LR. Molecular and immunohistochemical testing in mesothelioma and other mesothelial lesions. Arch Pathol Lab Med. 2024;148:e77–e89.
Ruegenberg S, Horn M, Pichlo C, Allmeroth K, Baumann U, Denzel MS. Loss of GFAT-1 feedback regulation activates the hexosamine pathway that modulates protein homeostasis. Nat Commun. 2020;11:687.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Sig Transduct Target Ther. 2020;5:209.
Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res. 2012;18:598–604.
Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, et al. Modification of RelA by O - linked N -acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc Natl Acad Sci USA. 2012;109:16888–93.
Liu J-R, Xu G-M, Shi X-M, Zhang G-J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci Rep. 2017;7:11698.
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–24.
Wang K, Ni L, Wang S, Zheng C. Herpes Simplex Virus 1 Protein Kinase US3 Hyperphosphorylates p65/RelA and Dampens NF-κB Activation. Hutt-Fletcher L, editor. J Virol. 2014;88:7941–51.
Silva JF, Olivon VC, Mestriner FLAC, Zanotto CZ, Ferreira RG, Ferreira NS, et al. Acute Increase in O-GlcNAc improves survival in mice with LPS-induced systemic inflammatory response syndrome. Front Physiol. 2020;10:1614.
Dong X, Shu L, Zhang J, Yang X, Cheng X, Zhao X, et al. Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-κB signaling pathway. J Neuroinflamm. 2023;20:146.
Zhang HR, Li TJ, Yu XJ, Liu C, Wu WD, Ye LY, et al. The GFPT2-O-GlcNAcylation-YBX1 axis promotes IL-18 secretion to regulate the tumor immune microenvironment in pancreatic cancer. Cell Death Dis. 2024;15:244.
Kim J, Lee HM, Cai F, Ko B, Yang C, Lieu EL, et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat Metab. 2020;2:1401–12.
Li N, Liu Q, Han Y, Pei S, Cheng B, Xu J, et al. ARID1A loss induces polymorphonuclear myeloid-derived suppressor cell chemotaxis and promotes prostate cancer progression. Nat Commun. 2022;13:7281.
Chen S, Jiang S, Zheng W, Tu B, Liu S, Ruan H, et al. RelA/p65 inhibition prevents tendon adhesion by modulating inflammation, cell proliferation, and apoptosis. Cell Death Dis. 2017;8:e2710.
Funding
This work was supported by the Postgraduate Research & Practice Innovation Programme of Jiangsu Province (SJCX23_0708), and Nanjing Medical University.
Author information
Authors and Affiliations
Contributions
QG, JL, and FZ designed the experiments. JW, SZ, and GC performed the experiments. JW and SZ performed the data analyses. JW and SZ wrote the manuscript. TC, YW and JZ provided technical support and sample tissues. QG, JL, and FZ organised and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conficts of interest.
Ethics approval and consent to participate
The study was approved by The Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2023-SR-502). All animal care and experimental procedures were conducted according to the National Research Council’s Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (IACUC-2308001).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, J., Zhou, S., Chen, G. et al. GFPT2: A novel biomarker in mesothelioma for diagnosis and prognosis and its molecular mechanism in malignant progression. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02830-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02830-4