Abstract
Background
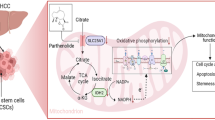
Liver cancer stem cells (LCSCs) significantly impact chemo-resistance and recurrence in liver cancer. Dopamine receptor D4 (DRD4) is known to enhance the cancer stem cell (CSC) phenotype in glioblastoma and correlates with poor prognosis in some non-central nervous system tumors; however, its influence on LCSCs remains uncertain.
Methods
To investigate the gene and protein expression profiles of DRD4 in LCSCs and non-LCSCs, we utilized transcriptome sequencing and Western blotting analysis. Bioinformatics analysis and immunohistochemistry were employed to assess the correlation between DRD4 expression levels and the pathological characteristics of liver cancer patients. The impact of DRD4 on LCSC phenotypes and signaling pathways were explored using pharmacological or gene-editing techniques. Additionally, the effect of DRD4 on the protein expression and intracellular localization of β-catenin were examined using Western blotting and immunofluorescence.
Results
DRD4 expression is significantly elevated in LCSCs and correlates with short survival in liver cancer. The expression and activity of DRD4 are positive to resistance, self renewal and tumorigenicity in HCC. Mechanistically, DRD4 stabilizes β-catenin and promotes its entry into the nucleus via activating the PI3K/Akt/GSK-3β pathway, thereby enhancing LCSC phenotypes.
Conclusions
Inhibiting DRD4 expression and activation offers a promising targeted therapy for eradicating LCSCs and relieve chemo-resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Almost all data generated or analysed during this study are included in this manuscript and additional files, while others are available from the corresponding author on reasonable request, except for the information related to patient privacy. RNA-Seq data generated in this study are available on Gene Expression Omnibus (GEO) with accession number GSE272591 at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272591.
References
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314.
Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10–24.
Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9:1331.
Garau L, Govoni S, Stefanini E, Trabucchi M, Spano PF. Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci. 1978;23:1745–50.
Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–97.
Rho SB, Kim BR, Kang S. A gene signature-based approach identifies thioridazine as an inhibitor of phosphatidylinositol-3’-kinase (PI3K)/AKT pathway in ovarian cancer cells. Gynecol Oncol. 2011;120:121–7.
Cheng HW, Liang YH, Kuo YL, Chuu CP, Lin CY, Lee MH, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6:e1753.
Dolma S, Selvadurai HJ, Lan X, Lee L, Kushida M, Voisin V, et al. Inhibition of dopamine receptor d4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29:859–73.
Akbari ME, Kashani FL, Ahangari G, Pornour M, Hejazi H, Nooshinfar E, et al. The effects of spiritual intervention and changes in dopamine receptor gene expression in breast cancer patients. Breast Cancer. 2016;23:893–900.
Campa D, Zienolddiny S, Lind H, Ryberg D, Skaug V, Canzian F, et al. Polymorphisms of dopamine receptor/transporter genes and risk of non-small cell lung cancer. Lung Cancer. 2007;56:17–23.
Liu Q, Zhang R, Zhang X, Liu J, Wu H, Li Y, et al. Dopamine improves chemotherapeutic efficacy for pancreatic cancer by regulating macrophage-derived inflammations. Cancer Immunol Immunother. 2021;70:2165–77.
Lee CJ, Baek B, Cho SH, Jang TY, Jeon SE, Lee S, et al. Machine learning with in silico analysis markedly improves survival prediction modeling in colon cancer patients. Cancer Med. 2023;12:7603–15.
Chen J, Wu S, Peng Y, Zhao Y, Dong Y, Ran F, et al. Constructing a cancer stem cell related prognostic model for predicting immune landscape and drug sensitivity in colorectal cancer. Front Pharm. 2023;14:1200017.
Yang Z, Wan W, Zhang P, Wang S, Zhao Z, Xue J, et al. Crosstalk between heat shock factor 1 and signal transducer and activator of transcription 3 mediated by interleukin-8 autocrine signaling maintains the cancer stem cell phenotype in liver cancer. J Gastroenterol Hepatol. 2023;38:138–52.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8.
Yanying W, Lei H, Ying D, Pingping Z, Guanling H, Jianjun L, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–25.
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9.
Amable G, Martinez-Leon E, Picco ME, Di Siervi N, Davio C, Rozengurt E, et al. Metformin inhibits beta-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol. 2019;112:88–94.
Soutto M, Peng D, Katsha A, Chen Z, Piazuelo MB, Washington MK, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–39.
Bekaii-Saab T, El-Rayes B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer Am Cancer Soc. 2017;123:1303–12.
Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11:22.
Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4.
Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–54.
Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702–10.
Zahalka AH, Estape AA, Maryanovich M, Nakahara F, Cruz CD, Finley L, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6.
Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–12.
Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34.
Wick MM, Kramer RA, Gorman M. Enhancement of L-dopa incorporation into melanoma by dopa decarboxylase inhibition. J Invest Dermatol. 1978;70:358–60.
Wen YT, Wu AT, Bamodu OA, Wei L, Lin CM, Yen Y, et al. A novel multi-target small molecule, LCC-09, inhibits stemness and therapy-resistant phenotypes of glioblastoma cells by increasing miR-34a and deregulating the DRD4/Akt/mTOR signaling axis. Cancers (Basel). 2019;11:1442.
Acknowledgements
We sincerely appreciate the contributions from the TCGA, GEO, GSEA, and HPA databases.
Funding
This work was supported by the Youth Fund of National Natural Science Foundation of China (Nos. 81803575 and 31902287), Key R&D and Promotion Projects of Henan Province (No.242102310467), Key Specialized Research and Promotion Project of Henan Province in 2023 (No. 232102311205), Henan Medical Science and Technology Research Program Project (No. LHGJ20210801), Program for Innovative Talents of Science and Technology in Henan Province (No. 23HASTIT043), College Students Innovation and Entrepreneurship Training Program of Henan University (No. 20231022007).
Author information
Authors and Affiliations
Contributions
ZY, PZ, YZ, RG, JH, QW, and HL participated in the experimental data collection; ZZ provided technical assistance; ZR designed experiments and drafted the manuscript; SL, YH, and DC helped revise the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experiments involving animal studies and patient specimens were reviewed and approved by the Ethics Committee of the Medical School of Henan University (HUSOM2023-221 and HUSOM2023-222). Informed consent was obtained from all participants involved in the study. All authors agree with the content of the manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Zhang, P., Zhao, Y. et al. DRD4 promotes chemo-resistance and cancer stem cell-like phenotypes by mediating the activation of the Akt/β-catenin signaling axis in liver cancer. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02811-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02811-7