Abstract
Background
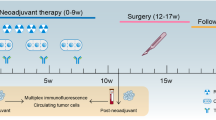
Preoperative chemoradiotherapy (CRT) followed by surgery is the standard treatment for locally advanced rectal cancer (LARC). We reported the short-term outcomes of the VOLTAGE trial that investigated the safety and efficacy of preoperative CRT followed by nivolumab and surgery. Here, we present the 3-year outcomes of this trial.
Methods
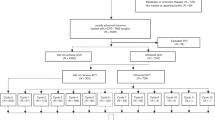
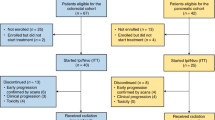
Thirty-nine patients with microsatellite stable (MSS) LARC and five patients with microsatellite instability-high (MSI-H) LARC underwent CRT (50.4 Gy) followed by five doses of nivolumab (240 mg) and surgery. The 3-year relapse-free survival (RFS), overall survival (OS), and associations with biomarkers were evaluated.
Results
The 3-year RFS rates in patients with MSS and MSI-H were 79.5% and 100%, respectively, and the 3-year OS rates were 97.4% and 100%, respectively. Of the MSS patients, those with pre-CRT PD-L1 positivity, pre-CRT high CD8 + T cell/effector regulatory T cell (eTreg) ratio, pre-CRT high expression of Ki-67, CTLA-4, and PD-1 had a trend toward better 3-year RFS than those without.
Conclusions
Three-year outcomes of patients with MSI-H were better than those of patients with MSS. PD-L1 positivity, elevated CD8/eTreg ratio, and high expression of Ki-67, CTLA-4, and PD-1 could be positive predictors of prognosis in patients with MSS.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT02948348.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EMK, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42.
Conroy T, Bosset JF, Etienne PL, Rio E, François E, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15.
Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: Long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8:e215445. https://doi.org/10.1001/jamaoncol.2021.5445.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–76.
Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: An open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023;8:422–31.
Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:38–48.
Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: Initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. 2021;7:1225–30.
Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin Cancer Res. 2022;28:1136–46.
Shamseddine A, Zeidan YH, El Husseini Z, Kreidieh M, Al Darazi M, Turfa R, et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat Oncol. 2020;15:233.
Bando H, Tsukada Y, Ito M, Yoshino T. Novel immunological approaches in the treatment of locally advanced rectal cancer. Clin Colorectal Cancer. 2022;21:3–9.
Seo I, Lee HW, Byun SJ, Park JY, Min H, Lee SH, et al. Neoadjuvant chemoradiation alters biomarkers of anticancer immunotherapy responses in locally advanced rectal cancer. J Immunother Cancer. 2021;9:e001610. https://doi.org/10.1136/jitc-2020-001610.
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767.
Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–84.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, e Sousa AHS Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004;240:711–8.
van der Valk MJM, Hilling DE, Bastiaannet E, Kranenbarg EMK, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch and Wait Database (IWWD): An international multicentre registry study. Lancet. 2018;391:2537–45.
Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. 2022;40:1681–92.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Shiraishi T, Sasaki T, Ikeda K, Tsukada Y, Nishizawa Y, Ito M. Predicting prognosis according to preoperative chemotherapy response in patients with locally advanced lower rectal cancer. BMC Cancer. 2019;19:1222.
Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8.
Wang Z, Shao C, Wang Y, Duan H, Pan M, Zhao J, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: A systematic review and meta-analysis. Int J Surg. 2022;104:106767.
Chalabi M, Fanchi LF, Dijkstra KK, den Berg JGV, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–76.
Rosner S, Reuss JE, Zahurak M, Zhang J, Zeng Z, Taube J, et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non-small cell lung cancer. Clin Cancer Res. 2023;29:705–10.
Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2022;17:909–20.
Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–36.
Acknowledgements
This investigator-initiated trial was funded by Ono Pharmaceutical Co. Ltd. We would like to express special thanks to all the participating patients, their families, all participating investigators, members of the Data and Safety Monitoring Board (Atsushi Sato [Hirosaki University], Keiji Koda [Teikyo University Chiba Medical Centre], and Koji Oba [Tokyo University]), and technical assistants (Miyuki Nakai, Chie Haijima, Megumi Hoshino, Kumiko Yoshida, Tomoka Takaku, Konomi Onagawa, Megumi Takemura, Yumi Osada, Miho Ozawa, and Yuka Nakamura).
Funding
The VOLTAGE study is an investigator-initiated investigational new drug (IND) clinical trial. A research grant and nivolumab were provided by Ono Pharmaceutical Co., Ltd. (Osaka, Japan).
Author information
Authors and Affiliations
Contributions
Y Tsukada: Conceptualization, Resources, Investigation, Visualization, Writing – original draft. H Bando: Conceptualization, Resources, Investigation, Writing – original draft. K Inamori: Resources, Investigation, Visualization. M Wakabayashi: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. Y Togashi: Investigation, Writing – review & editing. S Koyama: Resources, Writing – review & editing. D Kotani: Resources, Writing – review & editing. S Yuki: Resources, Investigation, Writing – review & editing. Y Komatsu: Resources, Investigation, Writing – review & editing. S Homma: Resources, Investigation, Writing – review & editing. A Taketomi: Resources, Investigation, Writing – review & editing. M Uemura: Resources, Investigation, Writing – review & editing. T Kato: Resources, Investigation, Writing – review & editing. M Fukui: Validation, Investigation, Writing – review & editing. N Nakamura: Resources, Investigation, Writing – review & editing. M Kojima: Resources, Investigation, Writing – review & editing. H Kawachi: Validation, Investigation, Writing – review & editing. R Kirsch: Validation, Investigation, Writing – review & editing. T Yoshida: Validation, Investigation, Writing – review & editing. A Sato: Data curation, Formal analysis, Project administration, Writing – review & editing. H Nishikawa: Investigation, Supervision, Writing – review & editing. M Ito: Conceptualization, Investigation, Supervision, Writing – review & editing. T Yoshino: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
HB has received research funding from AstraZeneca and Sysmex, and honoraria from Taiho Pharmaceutical and Eli Lilly Japan. MW has received honoraria from Nihon Medi-Physics Co., Ltd. YT has received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, AstraZeneca, and MSD; and research grants from Ono Pharmaceutical, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, AstraZeneca, KOTAI Biotechnologies Inc, and KORTUC outside this study. SK has received research funding from Otsuka Pharmaceutical and Chugai Pharmaceutical. DK has received honoraria from Takeda, Chugai, Lilly, MSD, Ono, Taiho, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, Novartis, Eisai, Seagen, Merck Biopharma, and Sysmex; research funding from Ono, MSD, Novartis, Servier, Janssen, IQVIA, Syneoshealth, Cimic, and Cimicshiftzero. SY has received honoraria from Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bayer Yakuhin Ltd., Bristol-Myers Squibb K.K., Takeda Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., MSD K.K., Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Daiichi Sankyo Co., Ltd. AT has received research grants from Taiho Pharmaceutical, Astellas Pharma, Takeda Pharmaceutical, Bayer Yakuhin, and Ono Pharmaceutical. AS has received research funding from Taiho Pharmaceutical, Boehringer Ingelheim, Bayer, Chugai Pharma, Eisai, Ono Pharmaceutical, Takeda, Aspyerian Therapeutics, Pentax Medical Devices, and Daiichi Sankyo/UCB Japan. HN has received research funding and honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, MSD, and Bristol-Myers Squibb, and research funding from Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Taiho Pharmaceutical, Oncolys BioPharma, Debiopharm, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside this study. HN also serves as a board member and founder of Sustainable Cell Therapeutics and CELLIAN-BiCLO outside this study. TY has received research grants from Amgen, Chugai, Daiichi Sankyo, Eisai, FALCO Biosystems, Genomedia Inc., Molecular Health, MSD, Nippon Boehringer Ingelheim, Ono, Pfizer, Roche Diagnostics, Sanofi, Sysmex, and Taiho, honoraria from Chugai Pharma, Takeda Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, and MSD K.K., and consulting fee from Sumitomo Corp.
Ethics approval and consent to participate
Written informed consent was obtained from all patients. The trial protocol was approved by the institutional review board of each participating site before the initiation of the study. The study was conducted in accordance with the tenets of the Declaration of Helsinki and Good Clinical Practice Guidelines after approval by the ethics board of each institution.
Consent for publication
Written informed consent for publication was obtained from the participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsukada, Y., Bando, H., Inamori, K. et al. Three-year outcomes of preoperative chemoradiotherapy plus nivolumab in microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Br J Cancer 131, 283–289 (2024). https://doi.org/10.1038/s41416-024-02730-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02730-7