Abstract
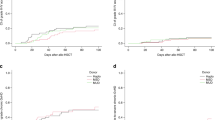
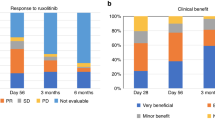
Posttransplant cyclophosphamide, sirolimus and mycophenolate mofetil (PTCy/siro/MMF) constitutes an innovative and well-tolerated acute graft-versus-host disease (aGVHD) prophylaxis after allogeneic stem cell transplantation (allo-HSCT), but risk factors for aGVHD incidence and therapy failure in this setting are scarce. This study prospectively registered all consecutive adult patients with hematologic malignancies who received a myeloablative allo-HSCT using PTCy/siro/MMF prophylaxis at our institution between 2017 and 2023. A total of 385 patients were included, of whom 44%, 34% and 22% were transplanted from matched sibiling, matched unrelated and haploidentical donors, respectively. The 180-day cumulative incidence of aGVHD was 21% (95% confidence interval [CI] 17–25%) for grade II–IV and 11% (95% CI 8–14%) grade III–IV aGVHD. The use of haploidentical donors was associated with an increased risk of severe aGVHD. Among 75 patients receiving first-line systemic corticosteroids, 49% achieved a sustained complete response, while 23% and 24% developed steroid-dependent (SD-aGVHD) and steroid-refractory aGVHD (SR-aGVHD), respectively. SR-aGVHD was associated with worse salvage treatment response and overall survival compared to SD-aGVHD. The 1-year cumulative incidence of aGVHD-related mortality was 5.4% (95% CI, 3.3–8.1). Risk factors for aGVHD-related mortality included haploidentical donors, older donors, diagnosis of myeldysplastic neoplasms, and grade IV aGVHD. This study confirms a low incidence aGVHD with PTCy/siro/MMF prophylaxis. SR-aGVHD showed poorer response to salvage therapies and worse survival, while haploidentical donors and older donor age were negative predictors for aGVHD-related deaths.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Anonymized data are available for researchers upon e-mail request to the corresponding author.
References
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:1–18. https://www.nature.com/articles/s41572-023-00438-1.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. https://pubmed.ncbi.nlm.nih.gov/18489989/.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9. https://pubmed.ncbi.nlm.nih.gov/19925877/.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811–7. https://pubmed.ncbi.nlm.nih.gov/28287639/.
Kanakry CG, Tsai HL, Bolaños-Meade J, Smith BD, Gojo I, Kanakry JA, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8. https://pubmed.ncbi.nlm.nih.gov/26764356/.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11. https://pubmed.ncbi.nlm.nih.gov/29544522/.
Kwon M, Bailén R, Pascual-Cascón MJ, Gallardo-Morillo AI, Sola AG, Balsalobre P, et al. Posttransplant cyclophosphamide vs cyclosporin A and methotrexate as GVHD prophylaxis in matched sibling transplantation. Blood Adv. 2019;3:3351–9. https://pubmed.ncbi.nlm.nih.gov/31698447/.
Sanz J, Galimard JE, Labopin M, Afanasyev B, Angelucci E, Ciceri F, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13. https://pubmed.ncbi.nlm.nih.gov/32375860/.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53. https://pubmed.ncbi.nlm.nih.gov/33140137/.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48. https://www.nejm.org/doi/full/10.1056/NEJMoa2215943.
Deeg HJ, Storb R, Thomas ED, Flournoy N, Kennedy MS, Banaji M, et al. Cyclosporine as prophylaxis for graft-versus-host disease: a randomized study in patients undergoing marrow transplantation for acute nonlymphoblastic leukemia. Blood. 1985;65:1325–34.
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35.
Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, et al. Calcineurin inhibitor-free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1828–34. https://pubmed.ncbi.nlm.nih.gov/25064745/.
Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Post-transplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan-based myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant. 2015;21:1506–14. https://pubmed.ncbi.nlm.nih.gov/26001696/.
Bejanyan N, Pidala JA, Wang X, Thapa R, Nishihori T, Elmariah H, et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv. 2021;5:1154–63. https://pubmed.ncbi.nlm.nih.gov/33635333/.
Greco R, Lorentino F, Albanese S, Lupo Stanghellini MT, Giglio F, Piemontese S, et al. Posttransplantation cyclophosphamide- and sirolimus-based graft-versus-host-disease prophylaxis in allogeneic stem cell transplant. Transpl Cell Ther. 2021;27:776.e1–776.e13. https://pubmed.ncbi.nlm.nih.gov/34087452/.
Elmariah H, Otoukesh S, Kumar A, Ali H, Arslan S, Shouse G, et al. Sirolimus is an acceptable alternative to tacrolimus for graft-versus-host disease prophylaxis after haploidentical peripheral blood stem cell transplantation with post-transplantation cyclophosphamide. Transpl Cell Ther. 2024;30:229.e1–229.e11. https://pubmed.ncbi.nlm.nih.gov/37952648/.
Montoro J, Luis J, Hernández-boluda JC, Hernani R, Lorenzo I, Pérez A, et al. Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors. Bone Marrow Transplant. https://doi.org/10.1038/s41409-020-0921-6.
Lazzari L, Balaguer-Roselló A, Montoro J, Greco R, Hernani R, Lupo-Stanghellini MT, et al. Post-transplant cyclophosphamide and sirolimus based graft-versus-host disease prophylaxis after allogeneic stem cell transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2022;57:1389–98. https://pubmed.ncbi.nlm.nih.gov/35680995/.
Chorão P, Henriques M, Villalba M, Montoro J, Balaguer-Roselló A, González EM, et al. Cytomegalovirus reactivations in allogeneic hematopoietic stem cell transplantation from HLA-matched and haploidentical donors with post-transplantation cyclophosphamide. Transpl Cell Ther. 2024;30:538.e1–538.e10. http://www.astctjournal.org/article/S2666636724001787/fulltext.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. https://pubmed.ncbi.nlm.nih.gov/19896087/.
Bacigalupo A, Milone G, Cupri A, Severino A, Fagioli F, Berger M, et al. Steroid treatment of acute graft-versus-host disease grade I: a randomized trial. Haematologica. 2017;102:2125.
Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–94. https://pubmed.ncbi.nlm.nih.gov/19001082/.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–59. http://www.thelancet.com/article/S2352302623003423/fulltext.
Asensi Cantó P, Sanz Caballer J, Fuentes Socorro C, Solves Alcaína P, Lloret Madrid P, Solís Ruíz J, et al. Role of extracorporeal photopheresis in the management of children with graft-vs-host disease. J Clin Apher. 2022;37:573–83. https://onlinelibrary.wiley.com/doi/full/10.1002/jca.22012.
Asensi Cantó P, Sanz Caballer J, Sopeña Pell-Ilderton C, Solís Ruiz J, Lloret Madrid P, Villalba Montaner M, et al. Real-world experience in extracorporeal photopheresis for adults with graft-versus-host disease. Transplant Cell Ther. 2023;29:765.e1–765.e8. https://pubmed.ncbi.nlm.nih.gov/37703997/.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–7.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. http://www.astctjournal.org/article/S1083879114013780/fulltext.
Hahn T, Sucheston-Campbell LE, Preus L, Zhu X, Hansen JA, Martin PJ, et al. Establishment of definitions and review process for consistent adjudication of cause-specific mortality after allogeneic unrelated-donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1679–86. https://pubmed.ncbi.nlm.nih.gov/26028504/.
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT—NIH—CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53:1401.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl J Med. 2020;382:1800–10. https://doi.org/10.1056/NEJMoa1917635.
Mohty M, Holler E, Jagasia M, Jenq R, Malard F, Martin P, et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood. 2020;136:1903–6. https://pubmed.ncbi.nlm.nih.gov/32756949/.
Ardizzoia F, Lorentino F, Bruno A, Marktel S, Giglio F, Clerici D, et al. Minnesota acute graft- versus-host disease risk score predicts survival at onset of graft- versus-host disease after post-transplant cyclophosphamide prophylaxis. Haematologica. 2022;107:2748–51. https://pubmed.ncbi.nlm.nih.gov/35899390/.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transpl [Internet]. 2012;18:1859–66. https://pubmed.ncbi.nlm.nih.gov/22863841/.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6. https://pubmed.ncbi.nlm.nih.gov/23423745/.
Sugita J, Kawashima N, Fujisaki T, Kakihana K, Ota S, Matsuo K, et al. HLA-Haploidentical Peripheral Blood Stem Cell Transplantation with Post-Transplant Cyclophosphamide after Busulfan-Containing Reduced-Intensity Conditioning. Biol Blood Marrow Transplant. 2015;21:1646–52. https://pubmed.ncbi.nlm.nih.gov/26093044/.
Ciurea SO, Zhang M, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2016;126:938–47.
Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, Digilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–47. https://doi.org/10.1182/blood-2015-09-671834.
Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131:2846–55. https://pubmed.ncbi.nlm.nih.gov/29545329/.
Axt L, Naumann A, Toennies J, Haen SP, Vogel W, Schneidawind D, et al. Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54:1805–14.
Zeiser R, Socié G, Schroeder MA, Abhyankar S, Vaz CP, Kwon M, et al. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): a randomised, multicentre, double-blind, phase 3 trial. Lancet Haematol. 2022;9:e14–25. https://pubmed.ncbi.nlm.nih.gov/34971577/.
Gooptu M, Romee R, St. Martin A, Arora M, Al Malki M, Antin JH, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138:273–82. https://pubmed.ncbi.nlm.nih.gov/34292325/.
Sanz J, Labopin M, Choi G, Kulagin A, Peccatori J, Vydra J, et al. Younger unrelated donors may be preferable over HLA match in the PTCy era: A study from the ALWP of the EBMT. Blood. 2024. https://pubmed.ncbi.nlm.nih.gov/38657278/.
Sanz J, Labopin M, Blaise D, Raiola AM, Busca A, Vydra J, et al. Haploidentical stem cell donor choice for patients with acute myeloid leukemia: a study from the ALWP of the EBMT. Blood Adv. 2024. https://pubmed.ncbi.nlm.nih.gov/38429091/.
Funding
Project “CM23/00215”, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union.
Author information
Authors and Affiliations
Contributions
PAC, IGS, MAS, AB and JS conceptualization and writing original draft. JM, MVM, PC, PSA, MSB, PLM, JSR, CSP, DMC, PGS, JEDR, AL, PR, PA, RB, and JDLR review and editing
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This project was approved by an ethical committee (Comité de Ética de la Investigación con Medicamentos – Hospital Universitario y Politécnico La Fe). Registry number: 09/2019-465. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asensi Cantó, P., Gómez-Seguí, I., Montoro, J. et al. Incidence, risk factors and therapy response of acute graft-versus-host disease after myeloablative hematopoietic stem cell transplantation with posttransplant cyclophosphamide. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02391-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02391-3