Abstract
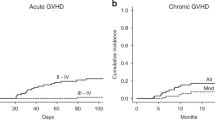
Anti-thymocyte globulin (ATG) has become a standard in preventing GVHD in related and unrelated donor transplantation, but there is no consensus on the best administration schedule. The PARACHUTE trial reported excellent CD4 immune reconstitution (CD4 IR) using a dosing schedule based on the patient’s weight and pre-conditioning absolute lymphocyte count (ALC). In 2015 we introduced the PARACHUTE dosing schedule for pediatric patients at our center. One hundred one patients were transplanted for malignant and non-malignant diseases. In this non-concurrent cohort CD4 IR+, defined by a single CD4 count >50/µL on day 90, was seen in 81% of patients. The incidence of grade II-IV and III to IV aGvHD was 26.6% and 15.3% and 5% for cGvHD with no severe cases. We found no difference in aGvHD between donor type and stem cell sources. Five-year EFS and OS were 77.5% and 83.5%. Grade III-IV GFRS was 75.2%. CD4 IR+ patients had better EFS (93.1% vs. 77.7%, p = 0.04) and lower non-relapse mortality (2.7% vs. 22.2%, p = 0.002). The PARACHUTE ATG dosing schedule individualized by weight and ALC results in good early immune reconstitution, low incidence of cGvHD, and favorable survival for patients with different disease groups, donor types, and stem cell sources.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during/or analyzed during the current study are not available due to data protection but are available from the corresponding author on reasonable request. Figures and tables are available on https://doi.org/10.6084/m9.figshare.22814654.
References
Bonifazi F, Rubio MT, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transpl. 2020;55:1093–102. https://doi.org/10.1038/s41409-020-0792-x.
Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015; https://doi.org/10.1016/j.bbmt.2014.11.676.
Yang X, Li D, Xie Y. Anti-Thymocyte Globulin Prophylaxis in Patients With Hematological Malignancies Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: An Updated Meta-Analysis. Front Oncol. 2021; https://doi.org/10.3389/fonc.2021.717678.
Finke J, Bethge WA, Schmoor C, Ottinger H, Stelljes M, Zander A, et al. ATG-Fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in hematopoietic cell transplantation from matched unrelated donors: a randomized, open-label, multicentre phase 3 trial. Lancet Oncol. 2009; https://doi.org/10.1016/S1470-2045(09)70225-6.
Shiratori S, Kurata M, Sugita J, Ota S, Kasahara S, Ishikawa J, et al. Graft-Versus-Host Disease Prophylaxis Using Low-Dose Antithymocyte Globulin in Peripheral Blood Stem Cell Transplantation-A Matched-Pair Analysis. Transplant Cell Ther. 2021; https://doi.org/10.1016/j.jtct.2021.08.029.
Kang HM, Kim SK, Lee JW, Chung NG, Cho B. Efficacy of low dose anti thymocyte globulin on overall survival, relapse rate, and infectious complications following allogeneic peripheral blood stem cell transplantation for leukemia in children. Bone Marrow Transplant. 2021; https://doi.org/10.1038/s41409-020-01121-9.
Locatelli F, Bernardo ME, Bertaina A, Rognoni C, Comoli P, Rovelli A, et al. Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomized, open-label, phase 3 trial. Lancet Oncol. 2017; https://doi.org/10.1016/S1470-2045(17)30417-5.
Bartelink IH, Belitser SV, Knibbe CA, Danhof M, de Pagter AJ, Egberts TC, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. 2013; https://doi.org/10.1016/j.bbmt.2012.10.010.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015; https://doi.org/10.1016/S2352-3026(15)00045-9.
Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016; https://doi.org/10.1182/blood-2016-06-721936.
Meesters-Ensing JI, Admiraal R, Ebskamp L, Lacna A, Boelens JJ, Lindemans CA, et al. Therapeutic Drug Monitoring of Anti-Thymocyte Globulin in Allogeneic Stem Cell Transplantation: Proof of Concept. Front Pharmacol. 2022; https://doi.org/10.3389/fphar.2022.828094.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Population pharmacokinetic modeling of Thymoglobulin(®) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. 2015; https://doi.org/10.1007/s40262-014-0214-6.
Admiraal R, Nierkens S, Bierings MB, Bredius RGM, van Vliet I, Jiang Y, et al. Individualised dosing of anti-thymocyte globulin in paediatric unrelated allogeneic haematopoietic stem-cell transplantation (PARACHUTE): a single-arm, phase 2 clinical trial. Lancet Haematol. 2022; https://doi.org/10.1016/S2352-3026(21)00375-6.
Admiraal R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017; https://doi.org/10.1016/j.jaci.2016.12.992.
Lakkaraja M, Scordo M, Mauguen A, Cho C, Devlin S, Ruiz JD, et al. Antithymocyte globulin exposure in CD34+ T-cell-depleted allogeneic hematopoietic cell transplantation. Blood Adv. 2022; https://doi.org/10.1182/bloodadvances.2021005584.
de Koning C, Prockop S, van Roessel I, Kernan N, Klein E, Langenhorst J, et al. CD4+ T-cell reconstitution predicts survival outcomes after acute graft-versus-host-disease: a dual-center validation. Blood. 2021; https://doi.org/10.1182/blood.2020007905.
Seo J, Shin DY, Koh Y, Kim I, Yoon SS, Min Byun J, et al. Association between preconditioning absolute lymphocyte count and transplant outcomes in patients undergoing matched unrelated donor allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning and anti-thymocyte globulin. Ther Adv Hematol. 2021; https://doi.org/10.1177/20406207211063783.
Woo GU, Hong J, Kim H, Byun JM, Koh Y, Shin DY, et al. Preconditioning Absolute Lymphocyte Count and Transplantation Outcomes in Matched Related Donor Allogeneic Hematopoietic Stem Cell Transplantation Recipients with Reduced-Intensity Conditioning and Antithymocyte Globulin Treatment.Biol Blood Marrow Transplant. 2020; https://doi.org/10.1016/j.bbmt.2020.06.005.
Heelan F, Mallick R, Bryant A, Radhwi O, Atkins H, Huebsch L, et al. Does Lymphocyte Count Impact Dosing of Anti-Thymocyte Globulin in Unrelated Donor Stem Cell Transplantation? Biol Blood Marrow Transplant. 2020; https://doi.org/10.1016/j.bbmt.2020.02.026.
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e260–e272. https://doi.org/10.1016/S1473-3099(19)30107-0.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56. https://doi.org/10.1016/j.bbmt.2005.09.004.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167. https://doi.org/10.1016/S2352-3026(19)30256-X.
Keesler DA, St Martin A, Bonfim C, Seber A, Zhang MJ, Eapen M. Bone Marrow versus Peripheral Blood from Unrelated Donors for Children and Adolescents with Acute Leukemia. Biol Blood Marrow Transplant. 2018; https://doi.org/10.1016/j.bbmt.2018.08.010.
Acknowledgements
We thankfully acknowledge Dr. Jan Jap Boelens for his guidance through this work and thoughtful review of the manuscript.
Author information
Authors and Affiliations
Contributions
FB and AW were responsible for the design and review of the protocol, extracting and analyzing data, and writing of the report. CSc was responsible for database management and analysis. PC, CSo, PZ, NA were responsible for gathering data and review of the manuscript. CV was responsible of reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barriga, F., Wietstruck, A., Schulze-Schiappacasse, C. et al. Individualized dose of anti-thymocyte globulin based on weight and pre-transplantation lymphocyte counts in pediatric patients: a single center experience. Bone Marrow Transplant 59, 473–478 (2024). https://doi.org/10.1038/s41409-024-02206-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02206-5