Abstract
Background
This study aimed to evaluate the histopathological concordance rates between prostate biopsies and radical prostatectomy specimens according to the applied biopsy approach (transrectal or transperineal).
Methods
We studied patients who had been newly diagnosed with clinically significant prostate cancer and who underwent a radical prostatectomy between 2018 and 2022. Patients were included if they underwent a prebiopsy magnetic resonance imaging and if they had not been previously treated for prostate cancer. Histopathological grading on prostate biopsies was compared with that on radical prostatectomy specimens. Univariable and multivariable logistic regression analyses were performed to assess the effect of the applied biopsy approach on histopathological concordance. Additional analyses were performed to assess the effect of the applied biopsy approach on American Urological Association risk group migration, defined as any change in risk group after radical prostatectomy.
Results
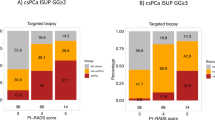
In total, 1058 men were studied, of whom 49.3% (522/1058) and 50.7% (536/1058) underwent transrectal and transperineal prostate biopsies, respectively. Histopathological disconcordance was observed in 37.8% (400/1058) of men while American Urological Association risk group migration was observed in 30.2% (320/1058) of men. A transperineal biopsy approach was found to be independently associated with higher histopathological concordance rates (OR 1.33 [95% CI 1.01–1.75], p = 0.04) and less American Urological Association risk group migration (OR 0.70 [95% CI 0.52–0.93], p = 0.01).
Conclusions
The use of a transperineal biopsy approach improved histopathological concordance rates compared to the use of a transrectal biopsy approach. A transperineal biopsy approach may provide more accurate risk stratification for clinical decision-making. Despite recent improvements, histopathologic concordance remains suboptimal and should be considered before initiating management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The research data for this study is available from the corresponding authors on reasonable requests.
References
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. Grading committee. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
van Leenders GJLH, van der Kwast TH, Grignon DJ, Evans AJ, Kristiansen G, Kweldam CF, et al. ISUP grading workshop panel members. The 2019 international society of urological pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44:e87–99.
Wenzel M, Würnschimmel C, Chierigo F, Mori K, Tian Z, Terrone C, et al. Pattern of Biopsy Gleason grade group 5 (4 + 5 vs 5 + 4 vs 5 + 5) predicts survival after radical prostatectomy or external beam radiation therapy. Eur Urol Focus. 2022;8:710–7.
Wenzel M, Würnschimmel C, Chierigo F, Flammia RS, Tian Z, Shariat SF, et al. Nomogram predicting downgrading in national comprehensive cancer network high-risk prostate cancer patients treated with radical prostatectomy. Eur Urol Focus. 2022;8:1133–40.
Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, Harewood L, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011;108:E202–10.
Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99.
Diamand R, Oderda M, Al Hajj Obeid W, Albisinni S, Van Velthoven R, Fasolis G, et al. A multicentric study on accurate grading of prostate cancer with systematic and MRI/US fusion targeted biopsies: comparison with final histopathology after radical prostatectomy. World J Urol. 2019;37:2109–17.
Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N. Engl J Med. 2020;382:917–28.
Goel S, Shoag JE, Gross MD, Al Hussein Al Awamlh B, Robinson B, Khani F, et al. Concordance between biopsy and radical prostatectomy pathology in the era of targeted biopsy: a systematic review and meta-analysis. Eur Urol Oncol. 2020;3:10–20.
Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg. 2005;75:48–50.
Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–4.
Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and “superbugs”: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114:384–8.
Zattoni F, Marra G, Kasivisvanathan V, Grummet J, Nandurkar R, Ploussard G, et al. The detection of prostate cancer with magnetic resonance imaging-targeted prostate biopsies is superior with the transperineal vs the transrectal approach. a European association of urology-young academic urologists prostate cancer working group multi-institutional study. J Urol. 2022;208:830–7.
Rai BP, Mayerhofer C, Somani BK, Kallidonis P, Nagele U, Tokas T. Magnetic resonance imaging/ultrasound fusion-guided transperineal versus magnetic resonance imaging/ultrasound fusion-guided transrectal prostate biopsy-a systematic review. Eur Urol Oncol. 2021;4:904–13.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–51.
de Rooij M, Israël B, Tummers M, Ahmed HU, Barrett T, Giganti F, et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur Radio. 2020;30:5404–16.
Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, et al. The FUTURE trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol. 2019;75:582–90.
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90.
Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–24.
Qu LG, Al-Shawi M, Howard T, Papa N, Poyet C, Kelly B, et al. Gleason grade accuracy of transperineal and transrectal prostate biopsies in MRI-naïve patients. Int Urol Nephrol. 2021;53:2445–52.
Marra G, Eldred-Evans D, Challacombe B, Van Hemelrijck M, Polson A, Pomplun S, et al. Pathological concordance between prostate biopsies and radical prostatectomy using transperineal sector mapping biopsies: validation and comparison with transrectal biopsies. Urol Int. 2017;99:168–76.
Evans SM, Patabendi Bandarage V, Kronborg C, Earnest A, Millar J, Clouston D. Gleason group concordance between biopsy and radical prostatectomy specimens: a cohort study from prostate cancer outcome registry - Victoria. Prostate Int. 2016;4:145–51.
Zattoni F, Marra G, Martini A, Kasivisvanathan V, Grummet J, Harkin T, et al. Is there an impact of transperineal vs. transrectal mri-targeted biopsy on the risk of upgrading in final pathology in prostate cancer patients undergoing radical prostatectomy? An EAU-YAU prostate cancer working group multi-institutional study. Eur Urol Focus. 2023;9:621–8.
Mai Z, Zhou Z, Yan W, Xiao Y, Zhou Y, Liang Z, et al. The transverse and vertical distribution of prostate cancer in biopsy and radical prostatectomy specimens. BMC Cancer. 2018;18:1205.
Cowan T, Baker E, McCray G, Reeves F, Houlihan K, Johns-Putra L. Detection of clinically significant cancer in the anterior prostate by transperineal biopsy. BJU Int. 2020;126:33–7.
Hagens MJ, Luining WI, Jager A, Donswijk ML, Cheung Z, Wondergem M, et al. The diagnostic value of PSMA PET/CT in men with newly diagnosed unfavorable intermediate-risk prostate cancer. J Nucl Med. 2023;64:1238–43.
Hoeh B, Flammia R, Hohenhorst L, Sorce G, Chierigo F, Tian Z, et al. Up- and downgrading in single intermediate-risk positive biopsy core prostate cancer. Prostate Int. 2022;10:21–7.
Ranasinghe W, Reichard CA, Nyame YA, Sundi D, Tosoian JJ, Wilkins L, et al. Downgrading from Biopsy grade group 4 prostate cancer in patients undergoing radical prostatectomy for high or very high risk prostate cancer. J Urol. 2020;204:748–53.
Bullock N, Simpkin A, Fowler S, Varma M, Kynaston H, Narahari K. Pathological upgrading in prostate cancer treated with surgery in the United Kingdom: trends and risk factors from the British Association of Urological Surgeons Radical Prostatectomy Registry. BMC Urol. 2019;19:94.
Bulten W, Pinckaers H, van Boven H, Vink R, de Bel T, van Ginneken B, et al. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: a diagnostic study. Lancet Oncol. 2020;21:233–41.
De Luca S, Fiori C, Bollito E, Garrou D, Aimar R, Cattaneo G, et al. Risk of Gleason Score 3 + 4 = 7 prostate cancer upgrading at radical prostatectomy is significantly reduced by targeted versus standard biopsy. Minerva Urol Nefrol. 2020;72:360–8.
Mandel P, Wenzel M, Hoeh B, Welte MN, Preisser F, Inam T, et al. Immunohistochemistry for prostate Biopsy-impact on histological prostate cancer diagnoses and clinical decision making. Curr Oncol. 2021;28:2123–33.
Author information
Authors and Affiliations
Contributions
Study concept and design: MH, LR, PVL, HVP; Statistical Analysis: MH, LR, VVN; Interpretation: MH, LR, PVL, HVP; Initial drafting of manuscript: MH, LR; Critical review: AJ, HV, KB, BB, RDB, AC, ML, MS, TR, SR, MS, SW, AV, PVL, HVP.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hagens, M.J., Ribbert, L.L.A., Jager, A. et al. Histopathological concordance between prostate biopsies and radical prostatectomy specimens—implications of transrectal and transperineal biopsy approaches. Prostate Cancer Prostatic Dis 27, 312–317 (2024). https://doi.org/10.1038/s41391-023-00714-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-023-00714-x
This article is cited by
-
Best of 2023 in Prostate Cancer and Prostatic Diseases
Prostate Cancer and Prostatic Diseases (2024)